-

Blog Post How integrated thinking can help boost the energy transition
-

Video A road project uncovered a historical archaeology site and one family’s connection to it
-

Blog Post Flash floods: Predicting the most difficult type of flood risk
-

Blog Post Transportation planning strategies for the FIFA World Cup and major events
Ideas
Explore the trends, innovations, and challenges impacting the built and natural environments.
Publications
Trending Topics
-
Smart Cities
Explore how smart technologies are transforming urban life—mobility, utilities, buildings, public spaces, and more.
Explore -
Coastal Resilience
How can we respond to the impacts of climate change on our coastal communities?
Explore -
Sustainable & Resilient Design
Our environmental challenges are many, but design can provide opportunities for positive change.
Explore -
Design Technology
From AI to VR, digital technologies are transforming the design process.
Explore
All Ideas
-

Blog Post Large-scale redevelopment projects need to learn from small-scale historic neighborhoods
-

Publication Research + Benchmarkeing Issue 04 | Inclusion, Diversity, and Wellness
-

Blog Post Net zero building design starts with industry climate pledges
-

Published Article How virtual reality could transform architecture
-

Video Innovation Insights: How can you effectively deal with market disruption in your industry?
-
Podcast The Construction Record Podcast™–Episode 342–Stantec’s Jag Singh on small modular reactors
-

Published Article Biden CO2, hydrogen pipeline dreams get wake-up call
-

Video Designing sustainability into Edmonton's LRT expansion
-
Published Article Four considerations for large pump station design
-

Blog Post Low-carbon energy solutions for mine sites
-

Published Article XR Marks the Spot: Using extended reality to propel innovation
-

Blog Post Preparing for building performance standards in cities
-

USSD 2024 – Stantec Presentation Schedule
-

Video How we decarbonize the mining industry
-

Blog Post How integrated thinking can help boost the energy transition
-

Technical Paper At the intersections of history
-

Publication Design Quarterly Issue 21 | Adaptive Reuse
-

Video Early childhood education in the modern world
-

Blog Post A sustainability roadmap is key in food manufacturing facility scale-up projects
-

Video A road project uncovered a historical archaeology site and one family’s connection to it
-

Blog Post Transportation planning strategies for the FIFA World Cup and major events
-

Blog Post Flash floods: Predicting the most difficult type of flood risk
-

Published Article What's the hype around machine learning and AI for flood mitigation?
-

Blog Post A single electricity market can increase affordability and reliability in Africa
-

Podcast Stantec.io Podcast: The Energy Transition
-

Blog Post 6 tips for designing allied health education facilities
-

Webinar Recording Navigating the storm: Solutions for automation and innovations in operational technology
-

Video Innovation Insights: How can artificial intelligence and data transform the AEC industry?
-

Blog Post Edge data centers can speed your online experience
-

Video Acoustics: Impact of noise and vibration
-

Conference NASTT 2024 No-Dig Show—Stantec Presentation Schedule
-

Blog Post Driving decarbonization with renewable floating offshore wind energy
-

Webinar Recording No H2 without H2O
-

Blog Post 8 ways to cool a factory
-

Video Interest is growing for recovering resources from wastewater
-
Video Prioritizing environmental justice on a $3 billion transportation megaproject
-

Published Article Mine by design
-

Blog Post Canada PFAS in the mining industry: Potential impacts, risks, and challenges
-

Blog Post A sustainable approach to readying critical minerals for decarbonization
-

Published Article Tech transformation: An inflection point for consultative engineering
-

Conference 2024 WEF Collection Systems and Stormwater Conference—Stantec Presentation Schedule
-

Blog Post Designing a workplace experience to answer: ‘Why should I go into the office?’
-

Video How Albany converted an underused highway ramp into a city park
-

Blog Post Can we recycle captured carbon to produce green hydrogen and biomass?
-

Published Article 3 things to consider when creating a lead service line inventory
-

Published Article Live, work, play: Office building conversion practices for downtown revitalization
-

Blog Post Small modular reactors: Driving energy security with nuclear power
-

Published Article Denver Water increasing height of Gross Reservoir Dam
-

Podcast Stantec.io Podcast: Advanced Tech and Reality Insight
-

Published Article Franklin Expedition shipwrecks in the Arctic face a new threat—melting ice
-

Video Innovation Insights: How does innovation impact the AEC industry?
-

Blog Post Could ‘smart’ building facades heat and cool buildings?
-

Published Article Towering transformation: Yale revamp of historic structure embraces green design
-

Blog Post Sustainable mining finances: Exploring costs and liabilities
-
Blog Post What is a comprehensive plan? 6 steps to help your city
-

Published Article Sense of Community: Culturally responsive design fits buildings to place and purpose
-

Webinar Stantec Water Webinar Series – 2024 Presentation Schedule
-

Video Behind the Scenes: Designing for Career and Technical Education
-

Blog Post Geoexchange system installation: 6 steps that prioritize safety and efficiency
-

Published Article What’s the deal with efficiency?
-

Blog Post 3 ways digital technology is pushing a revolution in the AEC industry
-

Webinar Recording Prepare for the Unexpected: Don’t let a flood put you under water
-

Publication Inside SCOPE at COP28
-

Blog Post The next destination: Passive design airports
-

Podcast Stantec.io Podcast: The Growth and Innovation Episode
-

Blog Post How do we secure the water supply needed for hydrogen production?
-

Video Raising dams to provide water security
-

Podcast Key water megatrends with Arthur Umble
-
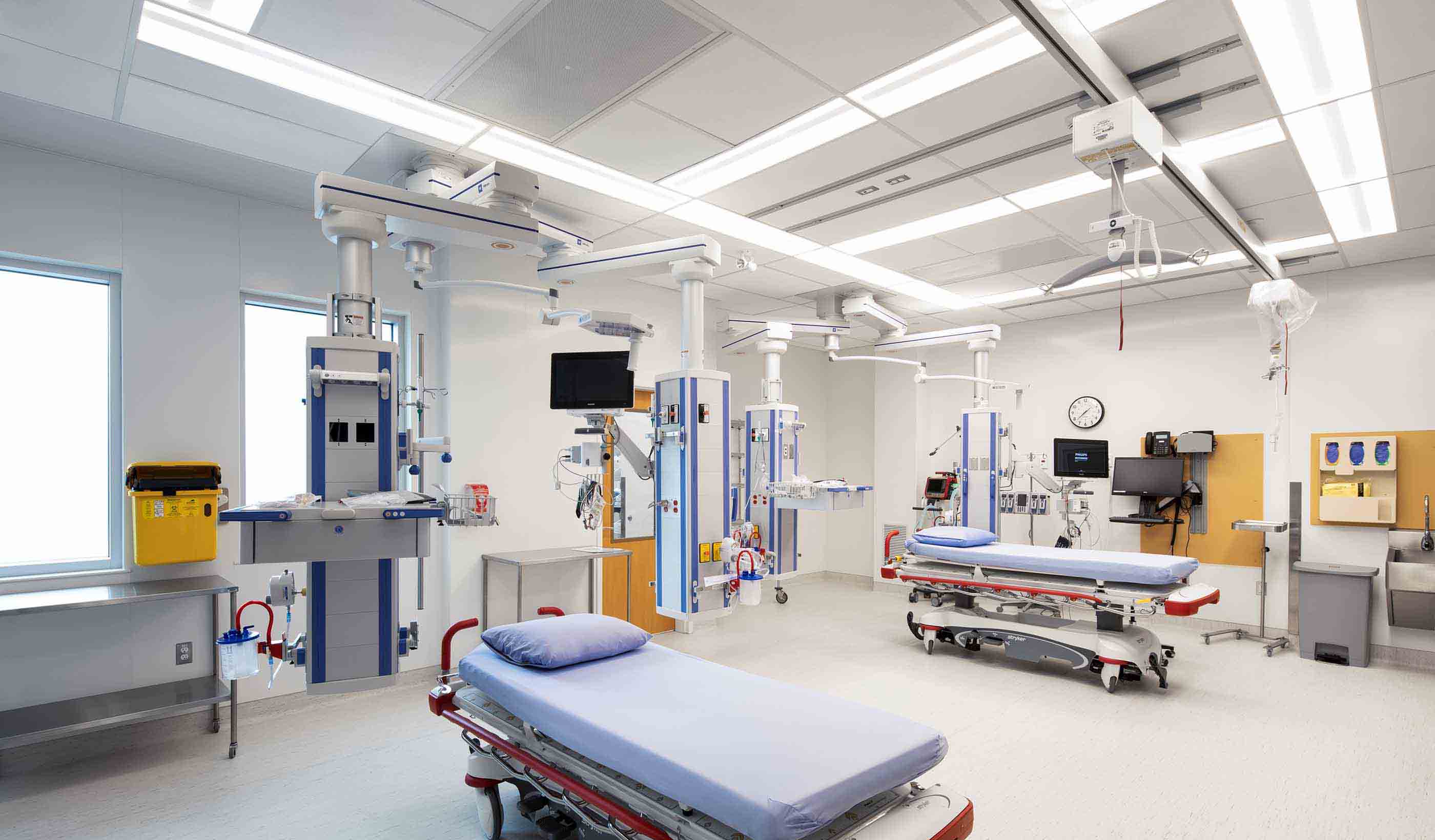
Blog Post Why decarbonizing hospitals smartly is better than electrification for healthcare design
-

Video Bringing a safe, reliable drinking water system back to Jackson, Mississippi
-

Blog Post Stream restoration requires working the bugs into your project
-

Blog Post Shrinking space: Post-pandemic law firm offices are smaller and more communal
-
Blog Post How green stormwater infrastructure can help cities manage intense rainfall events
-

Blog Post Building decarbonization strategy: It starts before electrification
-

Published Article Is your smart building an easy target for hackers?
-

Published Article Pumped storage in the USA: A story of IPPs, PPAs, and regulated utilities
-

Published Article The devastation of debris flows and the difficulty of predicting them
-

Published Article Optimizing complex HDD design and overcoming subsurface challenges
-

Blog Post Stantec’s Top 10 Ideas from 2023
-

Published Article Public agencies test digital twin tool for improved asset monitoring
-

Blog Post 3 steps to achieve One Water in the real world
-

Whitepaper Supporting healthier and more resilient communities through investments in mobility
-

Blog Post Does your building need a life cycle assessment?
-

Podcast Stantec.io Podcast: The Rail Episode
-

Report Showcasing success with Nature-based Solutions at COP28
-

Published Article The high stakes game
-

Published Article Stantec on mining’s quest for healthy closure
-

Blog Post Flood risk modeling using machine learning helps protect communities. Here’s how.
-

Blog Post Pumped storage hydropower and the Inflation Reduction Act are a renewable powerhouse
-

Published Article Sounding off on open office plans and workplace acoustics
-

Blog Post Low-carbon building materials: Designers discuss alternative options
-

Video How investing in downtowns fosters community and economic growth
-
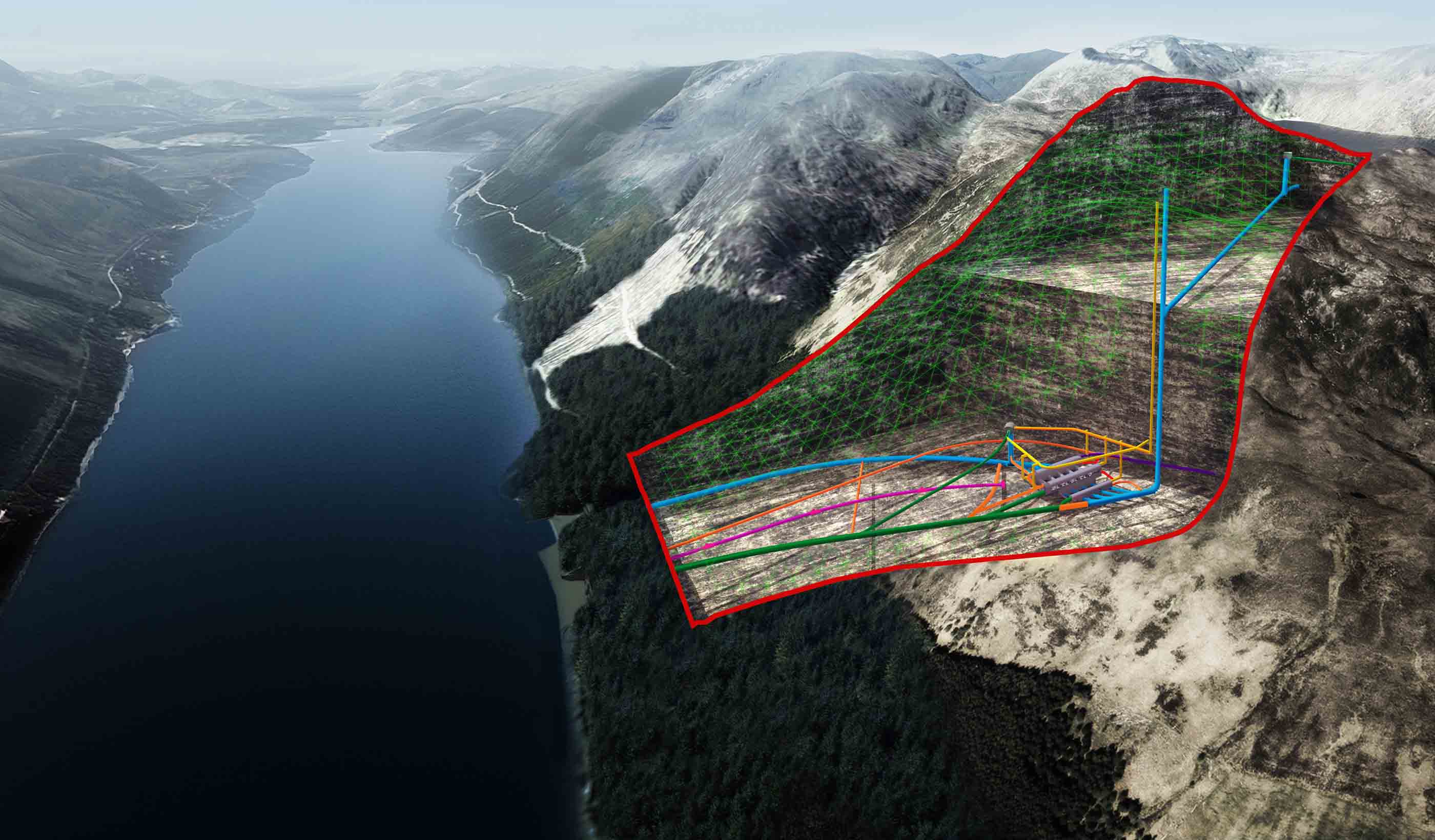
Published Article Pumped storage hydropower: Helping to drive the energy transition
-

Published Article Turquoise hydrogen producers could capture flourishing graphite market
-

Blog Post Dam removal: An engineer returns to restore a Connecticut river of his childhood
-

Whitepaper Adapting flood prediction for the climate crisis era
-

Publication Design Quarterly Issue 20 | RETHINK
-

Blog Post 5 questions to ask before you write a decarbonization RFP
-

Published Article Applying the 3Rs to mine ventilation
-

Report Catalyzing Calgary’s Downtown West
-

Published Article Protecting cultural artifacts: Archaeology’s role in building projects
-

Webinar Recording Design and development of large diameter water tunnels
-

Blog Post Mining decarbonization is key to creating clean energy
-

Blog Post New ASHRAE standards tip the balance toward net zero buildings
-

Webinar Recording Run your models faster and get results
-
Published Article Got yourself a deal? M&A lessons from Stantec
-

Blog Post Water for all: How to move past 3 big stressors
-

Video Restoring the coastal resilience of a unique Great Lakes ecosystem
-

Published Article Financing boom supercharges Superfund
-

Published Article Designing for school safety through CPTED and biophilic concepts
-

Video Miami design team sets the table for success
-

Webinar Recording Mall of the Future Webinar Series
-

Blog Post How to create a lead service line inventory through data
-

Video Helping meet tomorrow’s water needs in Colorado
-

Blog Post Soil carbon models: How remote sensing can measure the carbon stored in your soil
-

Published Article Hydraulic modeling approaches in drainage design and water resources engineering
-
Published Article Don’t 'debug' the selenium treatment system
-

Blog Post How does the 2020 National Building Code impact seismic design in Canada?
-

Blog Post Conceptual design: How a new digital tool helps buildings engineers work better
-
Podcast Talking Transit-Oriented Development with Sisto Martello
-

Video The Meaning of Design
-

Blog Post Design approaches for the next generation of EV battery manufacturing
-

Blog Post 4 questions a wastewater utility should ask before expanding
-

Blog Post Mobility hubs are the key to unlocking corridor development potential
-

Blog Post Dam safety: How technology can help dam owners and operators overcome 3 challenges
-

Blog Post How seahorse hotels protect endangered species in Australia
-

Blog Post What role do we play in decarbonization?
-

Blog Post Carbon capture methods: How can we capture and remove carbon from our atmosphere?
-

Blog Post 100% renewable energy: Making that commitment count
-

Podcast Stantec.io Podcast: The Dam Episode
-

Published Article Sustainably and quickly getting critical minerals to market
-

Blog Post So, you’ve captured carbon effectively. Now what?
-

Blog Post Embodied carbon: Mining to decarbonize buildings
-

Blog Post Reusing water while capturing carbon
-

Published Article Confidence in conveyance
-

Blog Post Passive house design regulations: Here are 8 strategies for multifamily projects
-

Video What is Stantec.io?
-

Podcast Scientists discover dinosaur ‘coliseum’ in Alaska’s Denali National Park
-

Technical Paper Post-wildfire debris flow and large woody debris transport modeling
-

Technical Paper Advancing debris flow hazard and risk assessments with modeling and rainfall intensity data
-

Published Article Communicating the difference between hazard and risk
-

Technical Paper What does landslide triggering rainfall mean?
-

Webinar Recording Mineral processing through a sustainability lens
-
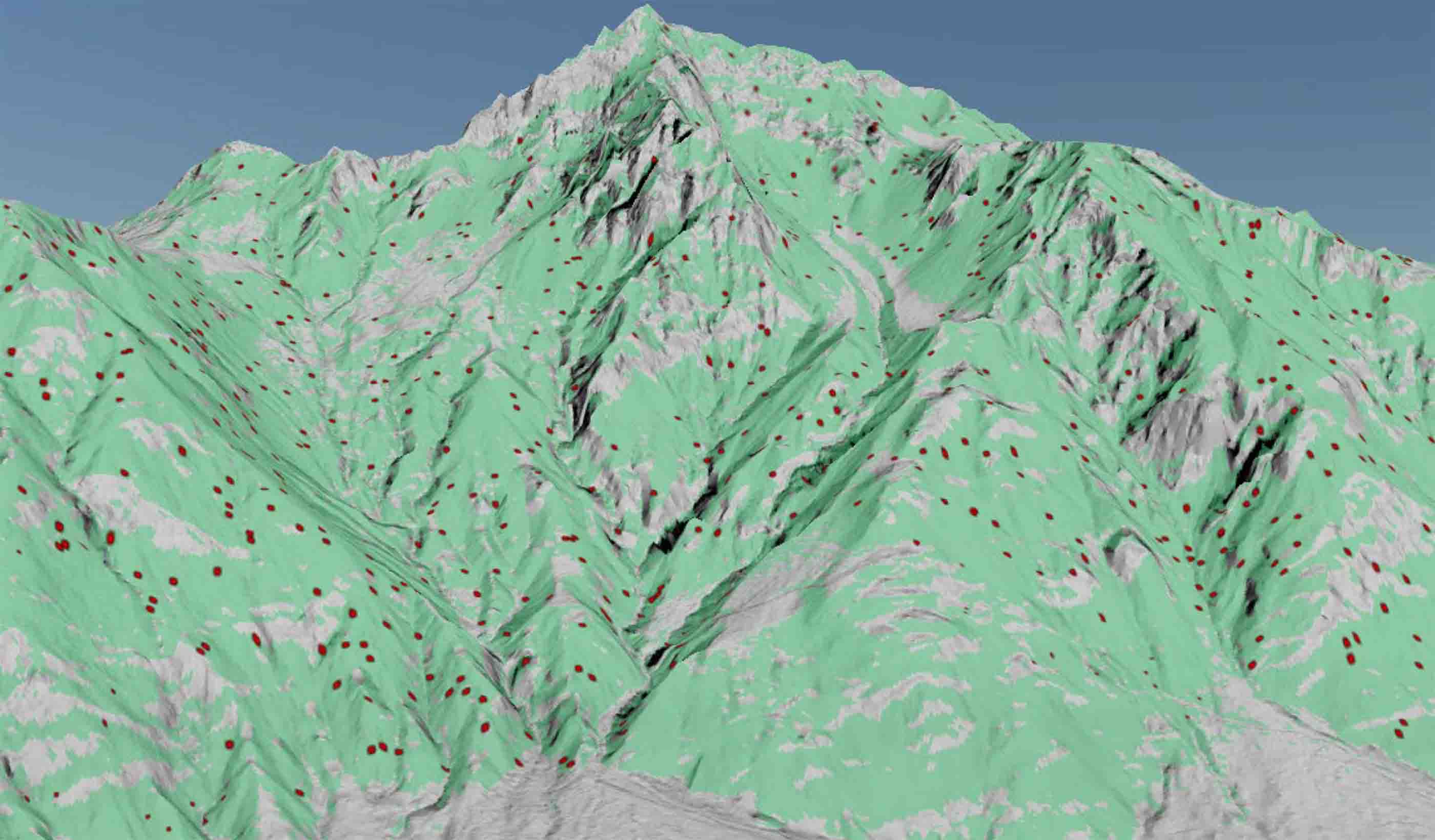
Technical Paper Debris flow hazard mapping along linear infrastructure: An agent based mode and GIS approach
-

Podcast Stantec.io Podcast: Extreme Weather and Digital Solutions
-

Published Article Floating offshore wind power could be the key to reaching decarbonization targets
-

Blog Post ESG programs that focus on health help build climate resilience, create financial benefits
-

Webinar Recording New US federal incentives will drive wastewater renewables
-

Video Helping create the most sustainable tree nursery business park in Europe
-

Blog Post How do you create thriving, connected places? 5 ways to expand transit-oriented planning
-

Video The costs behind PFAS treatment for drinking water
-

Blog Post The future of design and AI in architecture
-

Video Workplace Reboot: A workspace that reflects a commitment to sustainability, health, and well-being
-

Blog Post Robots in the workplace: This one scans for indoor air quality and more
-

Webinar Recording Optimizing blended water sources to prevent metal corrosion
-

Published Article Nature-based solutions: Crucial innovations for a resilient, sustainable future
-

Blog Post Solving for H: 4 challenges hydrogen experts are working to resolve
-

Published Article Addressing climate change
-

Design Quarterly Issue 19 | Decarbonization
-

Published Article Destination Maintenance
-

Published Article Developments in custody transfer metering of natural gas
-

Technical Paper Blind spots in CyanoHAB monitoring
-

Podcast How Stantec is using XR to propel innovation
-

Video Mitigating flooding impacts through stream restoration
-

Video A better way to move people and goods at airports? Autonomous vehicles
-

Video How can design better prepare future healthcare professionals?
-

Blog Post PFAS in drinking water: What utilities need to know about contaminants in water
-

Blog Post Advanced manufacturing facilities are key to a sustainable future
-

Video How drones are making bridge inspections safer and more efficient
-
Podcast Stantec.io Podcast: Stantec Beacon™
-
Published Article Canadian funding to advance mining’s ESG projects
-

Blog Post Stream restoration is critical for mine reclamation
-

Webinar Recording Potable reuse: Achieving water sustainability in a changing climate
-

Webinar Recording Be prepared for the unexpected
-

Published Article Turning tailings waste into value
-

Blog Post Inclusive student housing: The needs, numbers, and nuances
-

Whitepaper Rationalizing the SEC’s Enhanced Climate and ESG Disclosures
-

Blog Post Trauma-informed care: Indigenous healthcare designed for empathy, wellness, and safety
-

Published Article Integrated digital approach to managing geohazard risk
-

Published Article Analysis: Complexity and controversy cloud US permitting reform
-

Blog Post 5 key steps to put community at the center of transit and development
-
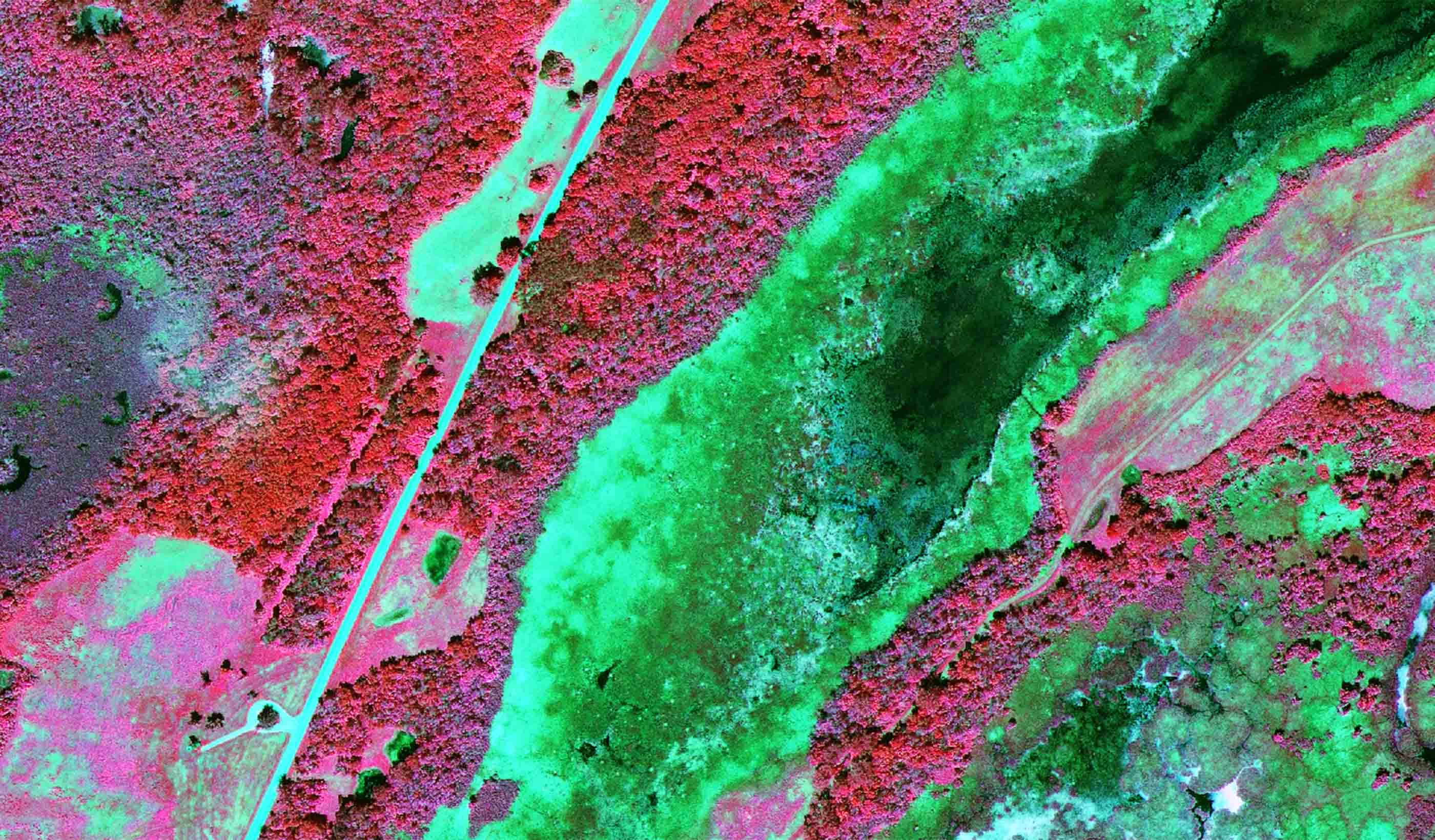
Published Article Detecting leaks using remote sensing pipeline monitoring
-

Published Article Master planning in an age of turbulent change
-

Blog Post Bats in buildings: How airborne eDNA can help you identify bat species at risk
-

Video Stantec Beacon™ and Travers (the largest solar facility in Canada)
-

Published Article Multi-pronged trenchless rehab approach
-

Blog Post Zero emissions buses and the energy transition: How do transit agencies adjust facilities?
-

Published Article How design in education is shaping workplaces of the future
-

Video Implementing AV Applications at Airports
-

Webinar Recording Get ahead of FEMA’s Future of Flood Risk Data Initiative
-

Blog Post How water utilities can develop a digital twin and use GIS mapping to meet future needs
-

Published Article Time for transformative thinking in western water
-

Video How to repeatedly save your client time and money with Stantec Beacon
-

Published Article Elevating community mental health
-

Published Article From A to Gen Z: Designing better workspaces for everyone
-

Report Real-world Examples of Biological Monitoring with Environmental DNA (eDNA)
-

Published Article Consultant Q&A: Stantec's Firas Al-Tahan on Evolution of Mobility, Design-Build
-

Blog Post 8 challenges holding back vertical farming facilities
-

Published Article Net zero potash mining
-

Webinar Recording The 2023 proposed revisions to the steam electric ELGs are out – now what?
-

Published Article Crews cut and roughen Colorado dam for $531M raise project
-

Video Improving transit safety and efficiency with the innovative Yard Control System
-

Video Retrofitting Hammond, IN’s downtown core from car-centric to a walkable neighborhood
-

Blog Post Don’t let construction ground your airport: 5 ways to manage traffic disruptions
-

Blog Post From microchips to canola oil, manufacturers want to build factories in North America
-

Blog Post A modular construction solution to the mental healthcare crisis
-

Blog Post How to fast-track climate change actions by using nature to reduce greenhouse gases
-

Blog Post Can emerging alternative delivery processes result in better hospital design?
-

Published Article Assessing, remediating and performance monitoring abandoned mine sites
-

Blog Post 3 steps to fully accessible and barrier-free transit
-

Blog Post 3 ways municipalities can use agile financial planning to prepare for unexpected events
-

Blog Post How is California tackling water sustainability with data? What does it mean elsewhere?
-

Blog Post Healthy roots: How to create educational spaces for early developmental success
-

Video Workplace Reboot: 4 ways buildings and property owners can decrease potential legislation penalties
-

Whitepaper How do environmental, social, and governance (ESG) and climate factors enhance valuations?
-
Webinar Recording Impacts of the new PFAS Maximum Contaminant Levels (MCL) and what you can do
-

Video Keeping communities connected through a modern bridge replacement
-

Published Article Drought management lessons from around the globe
-

Video Our underwater archaeology team explores Canada’s past
-

Published Article Wareham Dam interim spillway repairs
-

Published Article Paging All Future Veterinarians
-

Blog Post Fish passage: Fixing culverts is key to better stream habitat for salmon, other species
-

Published Article Emerging trend: Wind turbines paired with energy storage
-

Webinar Recording Design and operation of chloraminated water systems
-

Publication Design Quarterly Issue 18 | The Future of Design
-

Podcast Stantec.io Podcast: The GlobeWATCH Episode
-

Video What’s so great about integrated design?
-

Published Article How the latest technology makes better buildings
-

Blog Post Proton therapy design (Part 2): Tracing the patient journey
-

Blog Post Water affordability: How can utilities help their most vulnerable customers?
-

Published Article Evolving roles of the workplace designer
-

Blog Post How design can support mental health through crisis stabilization centers
-

Blog Post VR and community outreach programs: Building social acceptance with immersive technology
-

Published Article How does innovation in mining accelerate ESG goals?
-

Blog Post New York City’s Local Law 97 is fighting climate change, focusing on sustainable buildings
-

Blog Post PFAS in Canadian provinces: Where are the regulations?
-

Published Article Required: Clean Mine Power Solutions
-

Blog Post 6 design approaches that humanize cancer care amid technology advances
-
Published Article How to curb skepticism around Nature-based Solutions
-

Blog Post Across ever-changing landscapes, technology keeps our pipelines safe from geohazards
-

Blog Post 3 key steps to successful utility design for the telecommunications industry
-

Published Article The right ingredients for tailings management
-

Blog Post Designing for neurodiversity: Creating spaces that are inclusive of all
-

Blog Post How can machine learning help water utilities find lead service lines?
-

Blog Post Finding funding: New opportunities abound for the mining industry
-

Webinar Learn strategies to guide our communities in unlocking new urban opportunities
-

Blog Post Successful Indigenous school design: Listening, understanding, and acting
-

Video Growing native plants for better ecosystem restoration projects
-

Video Workplace Reboot: Understand the penalties for not complying with emissions legislation
-

Blog Post How can schools build strong career and technical education programs? Partnerships
-

Video Collaborating with communities to create stormwater solutions
-

Podcast Stantec.io Podcast: The Soil Risk Map Episode
-

Blog Post What is an innovation center? A collaborative workspace that’s essential in today’s office
-

Published Article Is your healthcare facility resilient enough?
-

Blog Post How prioritizing environmental justice in project planning can reduce discrimination
-

Blog Post Developing wastewater’s circular economy: How do we create a market?
-

Blog Post Desalination: Leveraging the potential of seawater
-

Blog Post Electrifying ferries can help us leverage crucial waterways while reducing emissions
-

Blog Post How does a mine site in the desert find water?
-

Blog Post Water and energy: A symbiotic relationship
-

Blog Post Water supply in the West
-

Blog Post Harnessing water to meet Canada’s renewable energy goals
-

Publication Research + Benchmarking Issue 03 | Planting Seeds
-

Blog Post Consider environmental due diligence as part of project planning—it can save time, money
-

Video Testing emerging AV technologies at airports
-

Published Article Development, Environment, Community: A Q&A with Stantec President and CEO Gord Johnston
-

Published Article Navigating the Complexity of the Hybrid Workplace Design Process
-

Blog Post Savings without sacrifice: Value engineering can counter inflation on projects
-

Podcast Stantec.io Podcast: The Smart Cities Episode
-

Blog Post Why biodiversity matters to your business after COP15
-

Published Article Net Positive Energy for People
-
Published Article Creating Better Spaces
-

Blog Post 5 trends from CES that will majorly impact the built environment
-

Report One Water, One Future: A promising water pricing model for equity and financial resilience
-
![[With Video] New city regulations are driving building retrofits](/content/dam/stantec/images/projects/0127/the-westory-181249.jpg)
Blog Post [With Video] New city regulations are driving building retrofits
-

Published Article The Mighty Bus: Transit Hero of Our Growing Community
-

Blog Post Debunking 7 myths and embracing a universal design mindset
-

Podcast Design Hive: Robyn Whitwham on designing for mental health
-

Blog Post Today’s parking lots are tomorrow’s mobility hubs. Why is it a better use of space?
-

Published Article Thinking Water: Challenges and opportunities of water management
-

Published Article A toilet in the middle of nowhere – with Derek Chinn
-

Published Article Expanding reservoirs to provide water security for communities
-

Published Article Net Zero: At the turning point
-

Published Article Tailings Management: A global concern
-

Published Article Challenges for the modernization of the Baygorria plant, Uruguay
-

Publication Design Quarterly Issue 17 | New Design Essentials
-

Published Article Mining EPCM Terms of Engagement
-

Blog Post Better than ever: 5 things becoming a parent taught me about pediatric design
-

Report Economic trends examined in Stantec Water’s new financial benchmarking report
-

Blog Post 7 ways we can design more resilient healthcare projects today
-
Report Delivering the Goods: Urban freight in the age of e-commerce
-

Podcast Stantec.io Podcast: The Digital Twin Episode
-

Published Article The challenges and opportunities of nature-based solutions
-

Blog Post Small modular reactors: How the next wave of nuclear power can fuel the energy transition
-

Video Workplace Reboot: How carbon impact legislation will affect building owners
-

Publication Inside SCOPE Issue 2: Week 2 at COP27
-

Blog Post When freshwater mussel surveys are needed, eDNA can show the way
-

Published Article Should interconnection participant funding be reformed or replaced?
-

Blog Post Bond programs 101: My school district needs money. Where do we start?
-

Blog Post Do you want functional lighting or fantastic lighting? The difference is commissioning
-

Publication Inside SCOPE Issue 1: Week 1 at COP27
-

Video Climate finance—leading change on an international scale
-
Report How data-informed mobility solutions create more equitable communities
-

Blog Post One module at a time: The future of affordable housing
-

Published Article Why healthcare masterplanning needs engineering masterminds
-

Blog Post 3 reasons why dam owners should prioritize adequate instrumentation and data
-

Published Article Global Hydropower Day: Stantec
-

Published Article Water in a Warming World
-

Published Article Nondestructive Testing: The key to bridge longevity
-
Blog Post Envisioning the future of your healthcare network: How to develop a campus master plan
-

Blog Post Adapting our green spaces for a changing climate
-

Podcast Stantec.io Podcast: The Community Engage Episode
-

Podcast Stantec.io Podcast: The Digital Strategy Episode
-

Blog Post Using well-being, student input, and stewardship to design a WELL school
-

Video What is COP?
-

Published Article Pumped storage advocates see bright future due to new tax credits, reliability needs
-

Published Article Why mine operators should conduct energy audits
-

Webinar Recording Sustainable mine closure and rehabilitation, responsibly caring for land
-

Published Article Small scale hydroelectric power plants used to possibly solve California's energy crisis
-

Published Article Nature-based protections against storm surges
-
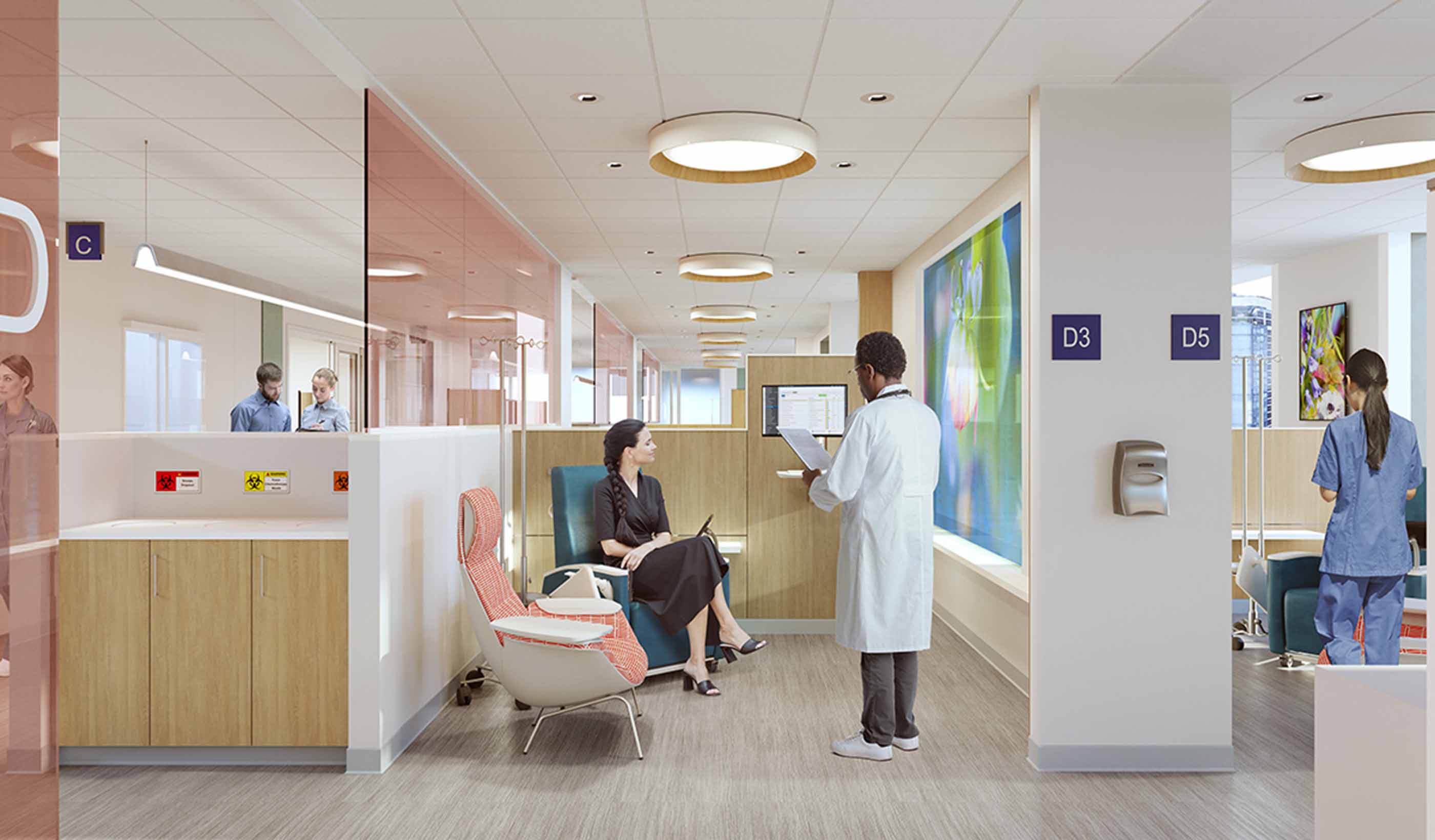
Blog Post Family’s cancer journey helps designer turn fragile moments into better projects
-

Published Article The Kenaitze Indian Tribe welcomes a new education center in Alaska
-
Podcast Digital frontiers, reimagining science, engineering, and design
-

Published Article The other shoe is dropping: Engineering solutions to over-sized carbon footprints
-

Published Article Up for the Challenge
-

Blog Post The rise of process intensification: Big gains with small footprints
-

Video Long Island Rail Road Third Track: expanding the busiest commuter line in the US
-

Published Article Pumped storage hydropower acts as a “water battery” that can sustainably power communities
-

Published Article US Offshore Wind Outlook
-

Video Cascading Climate Events and Predictions: Virtual Weather and Debris Flows
-

Video Building Decarbonization: The Urgency and Value
-

Video When is Solar Energy Green Enough
-

Video Cleaning Up the Mess: Quantifying Carbon Offsets with NbCS
-

Video How Remote Sensing Helps Call Out Climate Change Impacts Before Impact
-

Video It’s a Numbers Game: Using New Sensor Data to Achieve Climate Change Goals
-

Video Chasing Butterflies with our Eyes in the Sky
-

Video PipeWATCH: The Pipeline Protector From Outer Space
-

Video A Clear Lifesaver: Safeguarding water resources with Stantec’s WaterWATCH
-

Video Managing our Largest and Most Valuable Public Asset: Our Roadways
-

Video Measurements With Merit: Evolving Data Collection to Enable Integrated Decision-making
-

Video Optimizing a Zero Cost Energy Future for the Water Industry
-

Video Operations of the Future: Using Artificial Intelligence to Run a Water Treatment Facility
-

Video The Benefits of Getting Your Head in the Cloud with FAMS
-

Video How We’re Using Machine Learning to Deliver Rapid Probabilistic Flood Predictions
-

Video Smart(ER) Mobility Respects the Past with Future Thinking
-
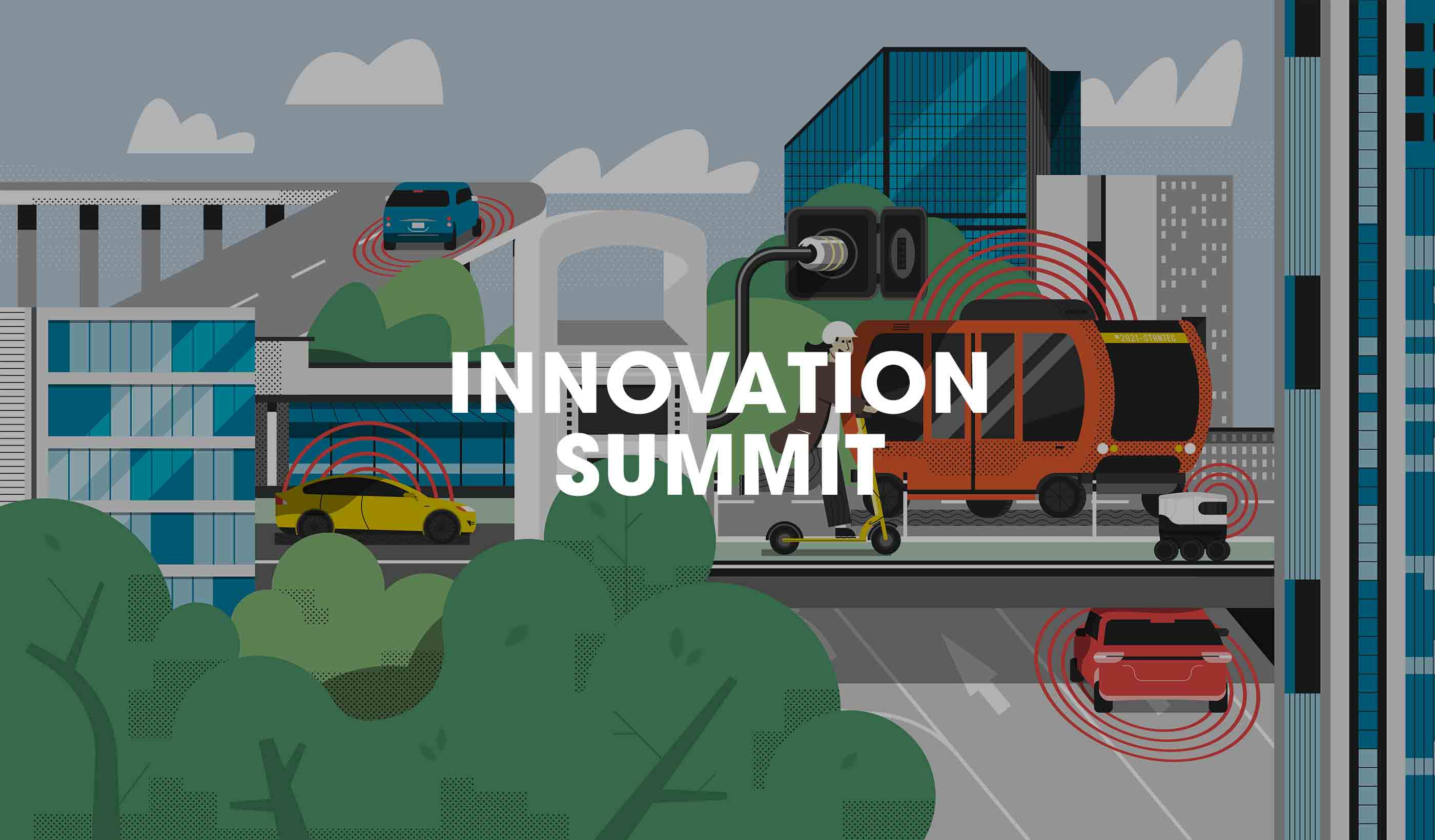
Video The Robots Are Coming. Are you ready?
-

Video Mapping the Route for a Zero-Emission Future
-

Video Buildings that Evolve: Design for Future-readiness
-

Video Digital Cities - Fire Flow
-

Video Planning a Smart City or Infrastructure? Don’t Start with Technology
-

Blog Post Bonn or bust: How can we get the most from limited climate finance?
-

Article Our guide to COP27
-

Published Article How mega events can be catalysts for change
-

Blog Post Designing for a client’s nature-driven mission
-

Report Equity in Stormwater Investments
-

Published Article Regulation Roundup: How to manage excess soil through “the pause” in Ontario
-

Blog Post 6 things wastewater treatment plant owners need to know about PFAS
-

Blog Post It takes a village: Managing water in a time of climate change
-

Blog Post How can utilities prepare for the next big storm?
-

Blog Post All about energy resiliency: How different countries are adapting to extreme weather
-

Blog Post Supply and demand: Energy security in the UK
-

Published Article Mitigating the social impacts of mining projects
-

Blog Post 7 big ideas for revitalizing the urban realm
-

Podcast Design Hive: Daryl Fonslow on adaptive reuse to net zero
-

Podcast Stantec.io Podcast: The FAMS Episode
-

Blog Post The mining energy crisis: Are the industry's net zero targets too ambitious?
-

Blog Post How can technology protect against extreme weather events?
-

Video Coming together to restore a Colorado treasure – the Alamosa River
-

Published Article Plugging offshore wind power into our energy grid
-

Blog Post Eastern promise: The compelling case for green hydrogen in Atlantic Canada
-

Video Workplace Reboot: Building consensus - a key (and fun!) aspect of workplace strategy
-

Blog Post Why Florida’s great untapped opportunity lies in its suburbs
-

Blog Post Measuring trees and tracking carbon sequestration from the sky
-
Video Stantec’s Buildings Team celebrates 10 years of being at the forefront of design
-

Published Article From necessity to amenity: changing the perception of storm water management
-

Podcast The SCOPE Episode 2: Sustainable Streets
-

Blog Post Energy efficiency: The first step in decarbonizing a mine
-

Blog Post Developing wastewater’s circular economy: How do we gain public acceptance?
-

Cleaner energy from waste
-

Blog Post Putting the people back in operations and maintenance facilities
-

Publication Design Quarterly Issue 16 | Climate Risk
-

Published Article UT Dallas Sciences Building Uses Zinc to Put Science on Display
-

Blog Post Gen Z water engineer: Climate change is the space race of my generation
-

Blog Post 5 steps companies can follow to change unused spaces into eye-popping pollinator habitat
-

Podcast Sustainability, Innovation and Failure to Launch
-

Published Article Woodlawn Cemetery could help solve mystery of Tampa’s erased Black cemeteries
-

Blog Post Rethinking air pressure in operating rooms could save lives
-

Technical Paper Simulation of some debris flows in Klanawa watershed in Vancouver, British Columbia
-

Video Workplace Reboot: A glass concourse transformed creates an urban, big city vibe
-

Published Article Brick-and-Mortar Retail Design: Reimagining the Way We Shop
-
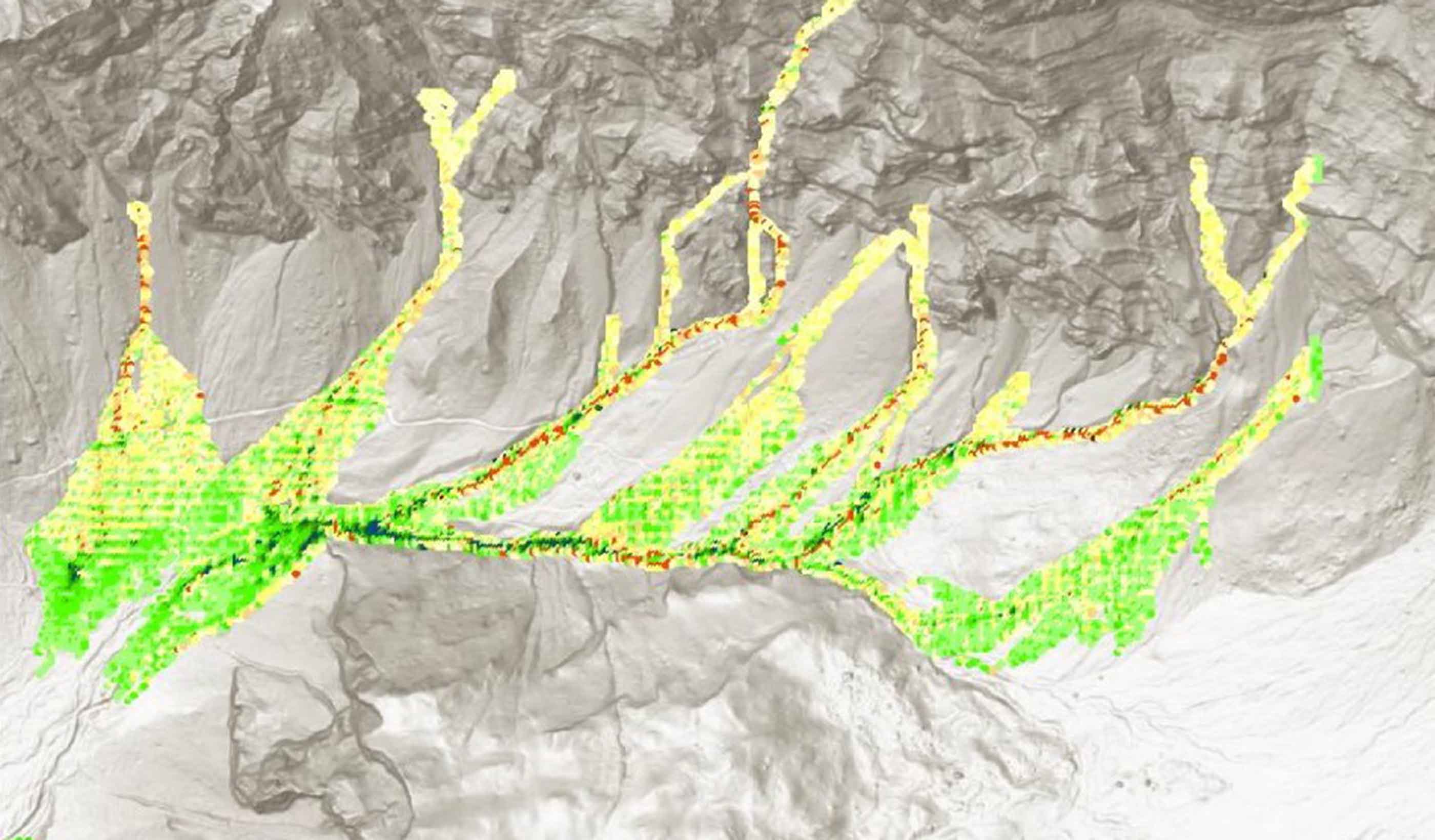
Technical Paper A comparison of two runout programs for debris flow assessment at the Solalex-Anzeindaz region of Switzerland
-

Published Article Air Control
-

Blog Post Even popular retail malls are reevaluating and refocusing to amplify experiences
-

Blog Post The future of the Cahill Expressway
-

Published Article Increasing capacity and funding for rural communities
-

Published Article Building an electric vehicle program: Where should cities start?
-

Our approach: The Climate Solutions Wheel
-
![[With Video] 7 approaches to creating affordable housing for artists](/content/dam/stantec/images/projects/0111/pullman-artspace-lofts-185195.jpg)
Blog Post [With Video] 7 approaches to creating affordable housing for artists
-

Published Article How high-performance buildings attract high-performing people
-

Podcast Stantec.io Podcast: The CommunityHQ Episode
-

Technical Paper Improving Environmental DNA Sensitivity for Dreissenid Mussels
-

Technical Paper Detecting bat environmental DNA from water-filled road-ruts in upland forests
-

Published Article Texas Stages Development of Statewide Flood Plan
-

Blog Post Designing for gender inclusivity in industrial facilities
-

Technical Paper Evaluating environmental DNA metabarcoding as a survey tool for unionid mussel assessments
-

Video Constructing a new park and transportation corridor on Manhattan’s shore
-

Published Article Riding the Risks and Opportunities of ESG in Mining
-
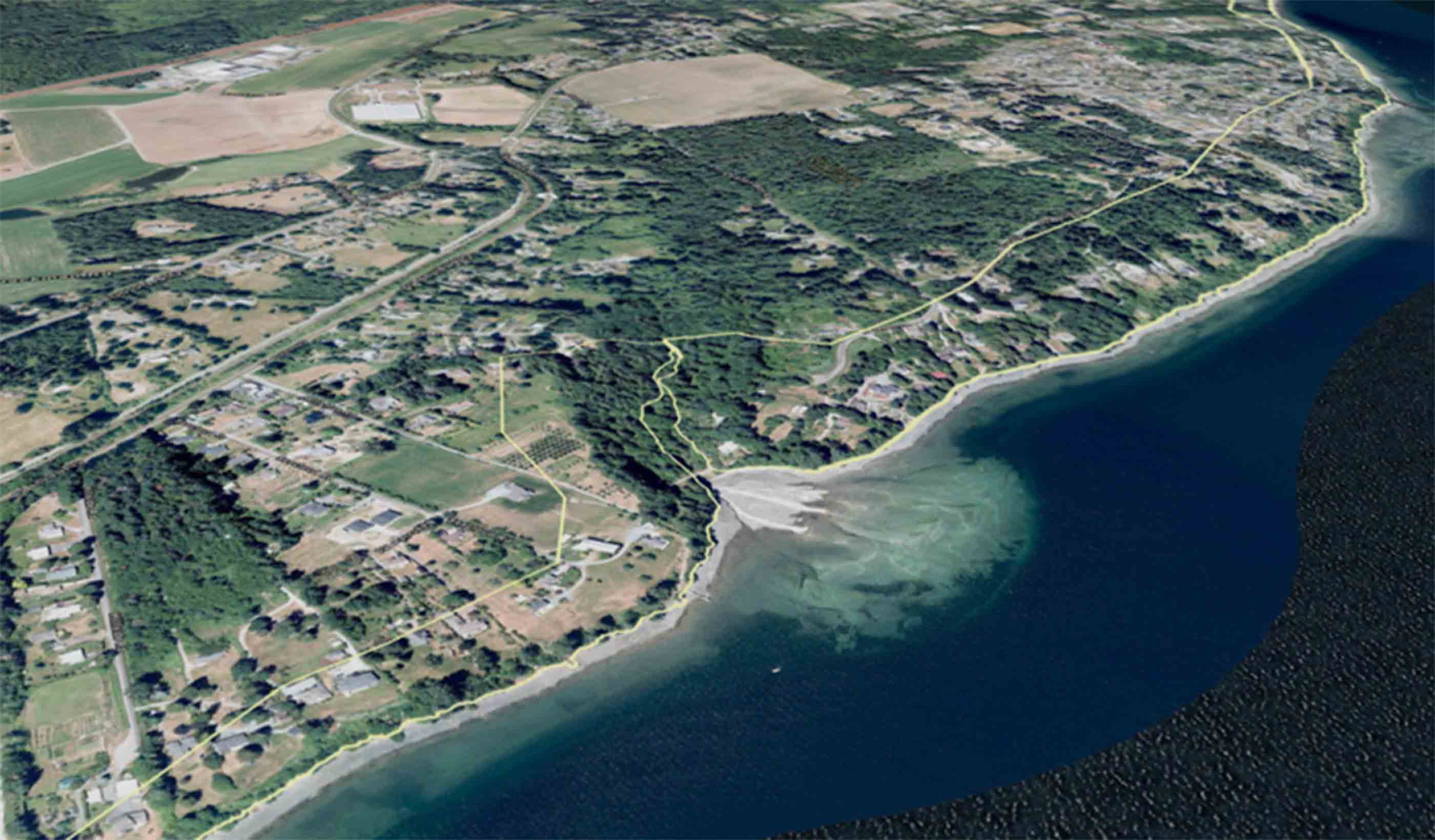
Presentation Saltair study guides development in areas that may be subjected to natural hazards
-

Blog Post Artificial intelligence and machine learning can diffuse water’s ticking time bomb
-
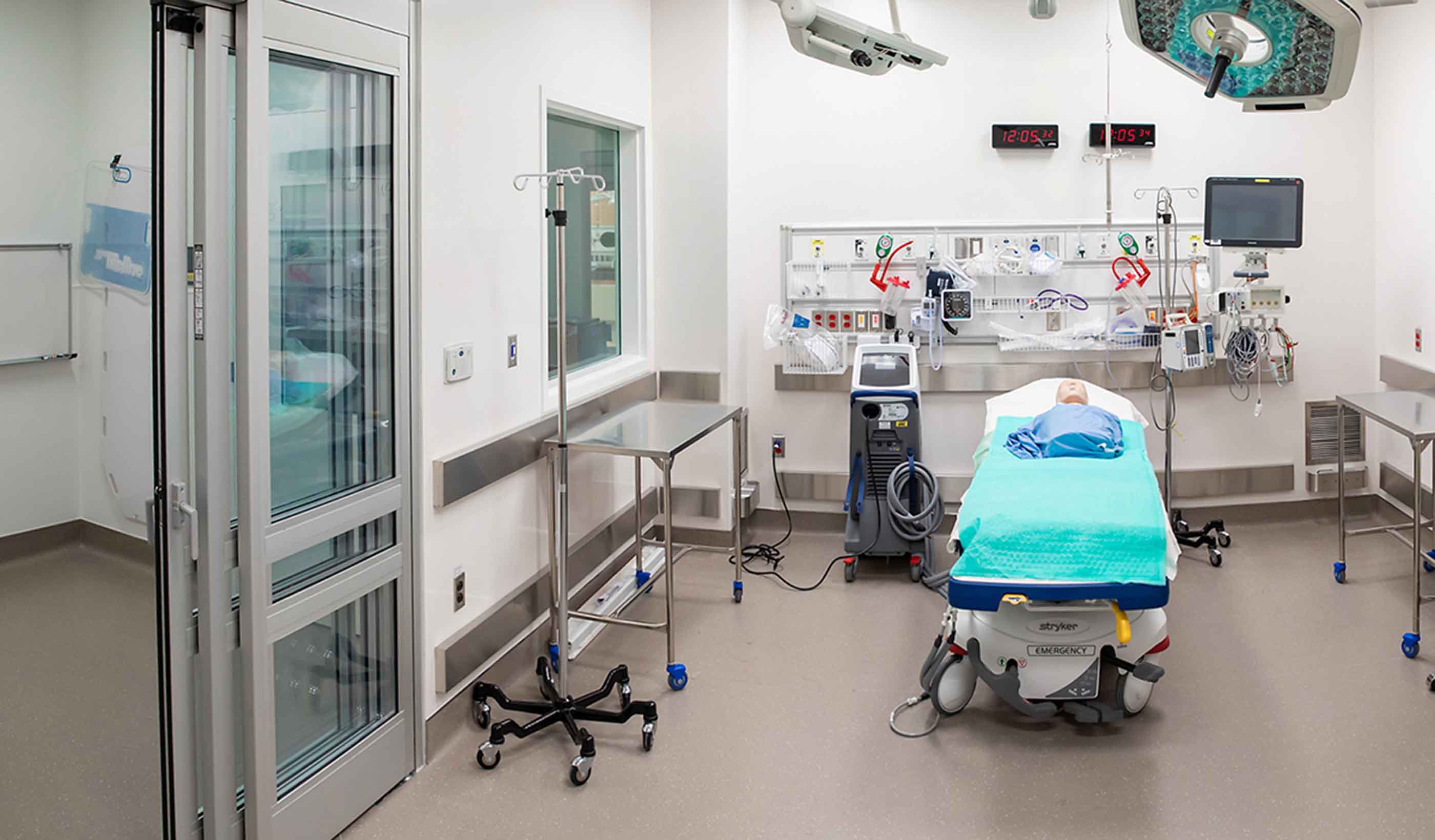
Blog Post Rethinking resuscitation rooms: A new approach for a vital healthcare space
-

Technical Paper Lessons learned from the local calibration of a debris flow model and importance to a geohazard assessment
-

Technical Paper Informing zoning ordinance decision-making with the aid of probabilistic debris flow modeling
-

Blog Post How can businesses and policy makers achieve climate justice?
-
Podcast Design Hive: Dr. Rick Huijbregts on smart cities
-

Blog Post Avoiding the wrecking ball: Restoring purpose in aging commercial properties
-

Video A new spin on an old bridge type for safer travel across Kentucky’s Green River
-

Blog Post 4 challenges to overcome when transmitting offshore wind power
-

Published Article Sustainability by design
-

Published Article Stronger Together: Indigenous Peoples and Mining
-

Blog Post Freshwater mussels 101: Explaining the “aquatic archaeology” behind mussel relocation
-

Blog Post 5 reasons why your company should address greenhouse gas emissions
-

Published Article Automated vehicles: Plan now, switch on later
-

Blog Post 7 approaches to building reuse to help existing properties reach net zero
-

Video Workplace Reboot: The challenge – modernize, relocate, expand, sustain
-
Podcast Stantec.io Podcast: The EnviroExplore Episode
-

Video Home is Where the Art is
-

Published Article A new era of downtown opportunity
-

Blog Post Proton therapy design (Part 1): Putting the patient experience first
-

Blog Post 4 ways to make sure your connected-infrastructure technology program is … well, connected
-

Published Article Modeling Post-Wildfire Debris Flow Erosion for Hazard Assessment
-

Published Article Retail, Restaurant Industries Embrace Post-Pandemic Design Shifts
-

Technical Paper Environmental genomics applications for environmental management activities
-

Published Article Using Environmental DNA (eDNA) to Improve Biological Assessments
-

Blog Post Buildings that care: 6 things to know about WELL certification in hospitals
-

Published Article A trip through British Columbia in the life of a shipping container
-
Blog Post Data + Design = Decision
-
Blog Post Are we finally about to achieve sustainable fusion energy?
-

Blog Post Can gaming influence human behavior when it comes to combating climate change?
-

Hydro batteries: Making renewables dispatchable
-

Blog Post What if simply restoring a closed mine site isn’t the best option?
-

Blog Post Can data improve dam safety?
-

How can solar canopies help us electrify our industry?
-

Published Article Treatment Trends
-

Blog Post What if the energy industry and environmentalists worked better together?
-

Blog Post For your next urban playground or revitalization, consider nature-based solutions
-

Blog Post Mapping your culture: How to help communities identify their heritage landmarks online
-

Blog Post A different West Side Story: How a boulevard changed Manhattan
-

Blog Post Breathe easier: Mitigating welding hazards with ventilation and exhaust
-

Blog Post Making smart asset choices from imperfect asset data
-

Blog Post Surveying Hawaii’s Kalaupapa Peninsula: Connecting a rich past to an exciting future
-

Blog Post Reflecting on Frederick Law Olmsted’s legacy for World Landscape Architecture Month
-
Podcast Stantec.io Podcast: The Route Selector Episode
-

Video Workplace Reboot: What’s so great about Beauty and Awe?
-

Blog Post The 4 pillars of ecosystem restoration work together to create better communities
-
![[With Video] 4 reasons we’re excited about our new automated design tool](/content/dam/stantec/images/ideas/blogs/021/audet-design-automation-graphic.jpg)
Blog Post [With Video] 4 reasons we’re excited about our new automated design tool
-

Published Article Shrink Your Carbon Footprint
-

Blog Post How two scientists collaborated to develop a tool for better stream restoration design
-

Blog Post Developing wastewater’s circular economy: How do we pay for it?
-

More Energy Independence in Indigenous Canada
-

Blog Post Urban highway removal: 4 ways to reknit a city’s fabric
-

Blog Post A new tool to protect marine environments from wastewater nutrients
-

Publication Design Quarterly Issue 15 | Reuse and Revitalization
-

Blog Post Designing living systems to adapt to a changing climate
-

Video A channel runs through it: How we addressed the challenges of urban creek restoration
-
Blog Post Reinventing the Thompson Center: A research project into a modular, mixed-use destination
-

Published Article Deeper mines are tantalizing but costly
-

Blog Post Arc flash hazards: How engineering and standards can make for safer electricity use
-

Blog Post 3 design solutions to sort out the carbon impact of logistics
-

Blog Post Groundwater plays a critical role in climate change adaptation
-

Video Workplace Reboot: A reimagined office building becomes a magnet for both employers and employees
-

Blog Post Creating memories in a supportive home away from home
-

Blog Post Why we all need to care about mining
-

Blog Post Is your infrastructure facing climate risks? Here’s a tool to assess that
-

Podcast Stantec.io Podcast: The Fire Flow Episode
-

Podcast What does the future hold for autonomous vehicle policy and funding?
-

Blog Post Museum lighting case study: How a single exhibit embodies an impactful mission
-

Blog Post Smart resilience planning and design includes triple bottom line benefits
-

Published Article The future is now
-

Published Article Urban highway removal projects are on the rise across America
-

Published Article Santiago Airport becomes the most modern in South America
-

Blog Post Breaking the bias: How two women are making spaces and places more inclusive
-

Published Article How to Plan Office Space When Temporary Becomes Permanent
-

Published Article 4 HVAC Strategies to Still Protect Against COVID-19
-

Published Article A Guide to Workplace Metrics, Data, and Research
-

Blog Post Water reuse and river flows: What can we learn from the LA River?
-

Technical Paper Pathways between climate, fish, fisheries, and management
-

Video Planning for the future of healthcare in Ontario
-

Blog Post The (remote) doctor is in: 5 ways telehealth is changing design
-

Podcast Mining Stock Daily: AME’s Roundup Day 3 Recap
-

Blog Post Design through stories: Experience-based design in pediatric healthcare
-

Video Workplace Reboot: There's more to lighting design than meets the eye
-

Published Article Combining Generic/Flexible Labs with Highly Specialized Research Space
-

Article Stantec’s letter to the US Department of Transportation on its 2022-26 strategic plan
-

Blog Post Resilience checklist: 10 steps to improving your community’s resilience
-

Publication Research + Benchmarking Issue 02 | Connected
-

Webinar Recording Climate Solutions Webinar Series: Climate solutions that work
-

Published Article Water analytics data helping the rise of smart utilities
-

Blog Post The new low-carbon suburb: Retrofitting communities for success in the post-COVID era
-

Podcast Stantec.io Podcast: The Connect Episode
-

Published Article It takes two: How architects and engineers blend their talents
-

Blog Post What is design automation, and how can you optimize it for your next project?
-

Webinar Recording Climate Solutions Webinar Series: Navigating climate change terminology
-

Video Workplace Reboot: Law firm takes the hybrid approach
-

Blog Post Railroad crossings: Involving locomotive engineers to better understand crash risks
-

Webinar Recording Climate Solutions Webinar Series: Why now, and why us?
-

An energized future
-

Published Article 2021 Trenchless Q&A: A Look at Trenchless Engineering in Canada
-

Published Article Smarter care at Vaughan’s first hospital
-

Published Article St. Petersburg, Florida: Coastal Resiliency and Community Sustainability
-

Technical Paper Assessing the use of a SWA for treating oil spills in Canada’s freshwater environments
-

Report Quick and creative street projects
-

Video From airside to landside: Diversifying the airport program
-

Blog Post How do we shift to a circular economy? It starts with dumping the end-of-life concept
-

Published Article The importance of the author-verifier relationship in project management
-

Podcast Design Hive: Brenda Bush-Moline on health equity
-

Technical Paper New research quantifies risk to bats at commercial wind facilities
-

Published Article Tailings Management, Meet Science Fiction
-

Published Article Screening and Framework Considerations for a PEL Approach
-

Webinar Recording Lateral structure jacking - how to install a railroad tunnel in a weekend
-

Report The Infrastructure Investment and Jobs Act: How Agencies Can Position for Funding
-

Blog Post We can build healthier streets by prioritizing the human experience
-

Webinar Recording What’s blowing your way? Proposed new odor guideline and monetary environmental penalties.
-

Blog Post 4 keys to keeping mechanical systems running during a hospital renovation
-
Published Article UV-C Radiation to combat healthcare associated infections
-

Blog Post How to use funding trends to promote healthier lifestyles and better public space
-

Blog Post Combating the zebra mussel: What you need to know
-

Blog Post 4 ways smart utilities improve water infrastructure
-

Video GlobeWATCH: World Leading Remote Sensing Technology
-

Webinar Recording Ontario’s New Soil Regulation - Panel Discussion
-

Published Article Model builders
-

Blog Post Children’s play area design: How landscape architects set the stage for fun and games
-

Published Article Community-minded Design
-

Video For our US Audience: If We Built it Today – The Hoover Dam
-

Blog Post First Nations school design case study: Listen, learn, then design
-

Published Article Delivering pumped hydro storage in the UK after a three-decade interlude
-

Publication Inside SCOPE Issue 2: Week 2 at COP26
-
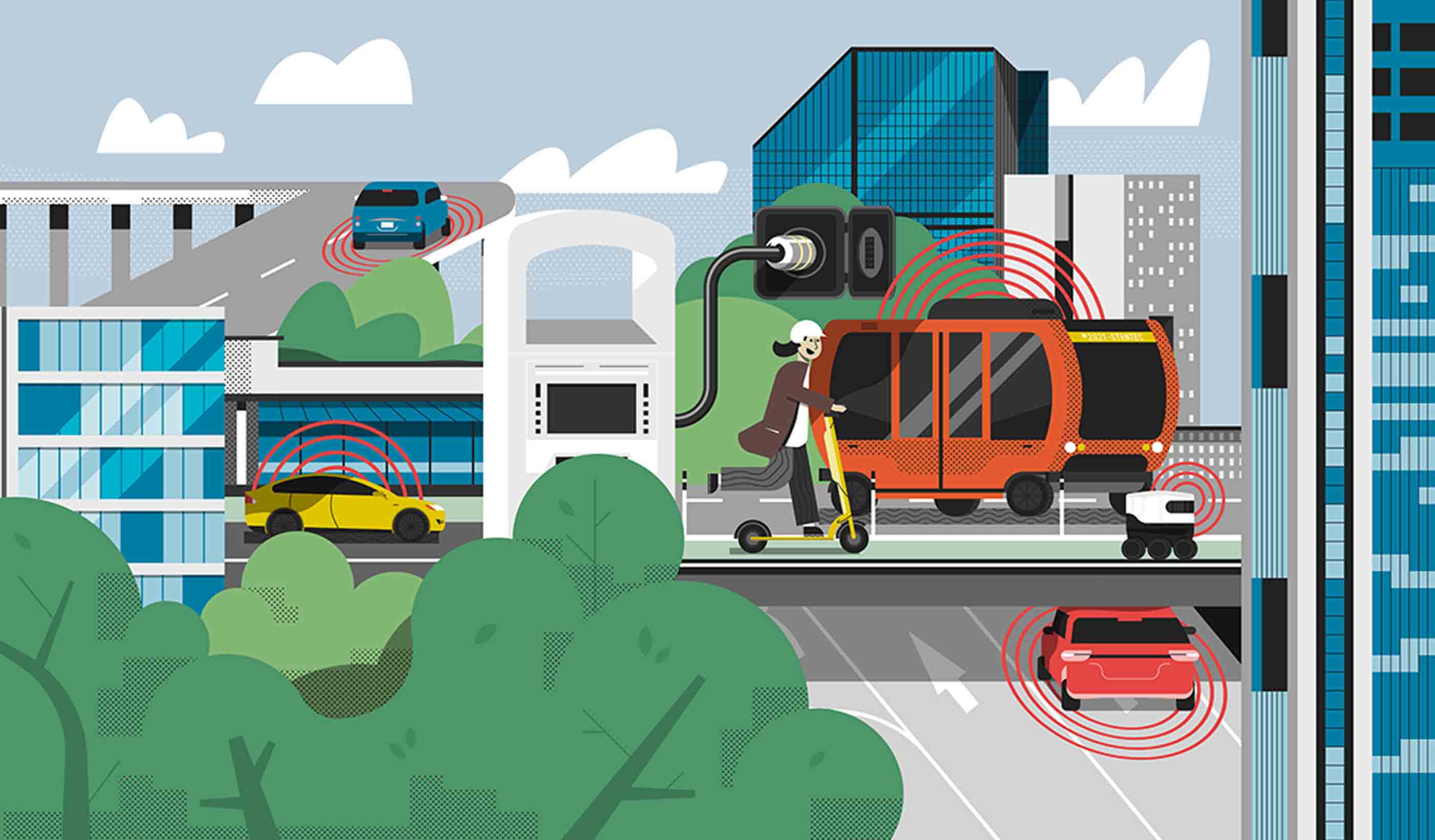
Blog Post EV is the bridge to transit’s AV revolution—and now is the time to start building it
-

Blog Post Managing the future: Why you should consider climate change risks on your next project
-

Blog Post Investigating PFAS: Key concerns for sampling programs
-

Technical Paper Aggradation, degradation in gravel-bed rivers mediated by sediment storage and morphologic
-

Publication Inside SCOPE Issue 1: Week 1 at COP26
-

Published Article No aspect of a mine will remain untouched
-

Blog Post A Smart(ER) approach to mobility: Prioritizing equity and resilience
-

Publication Design Quarterly Issue 14 | Tools and Data
-

Blog Post Student housing success: How P3 helped UC Davis meet its goals
-

Blog Post Brownfields, synchronized: Pros and cons of aligning development goals with remediation
-
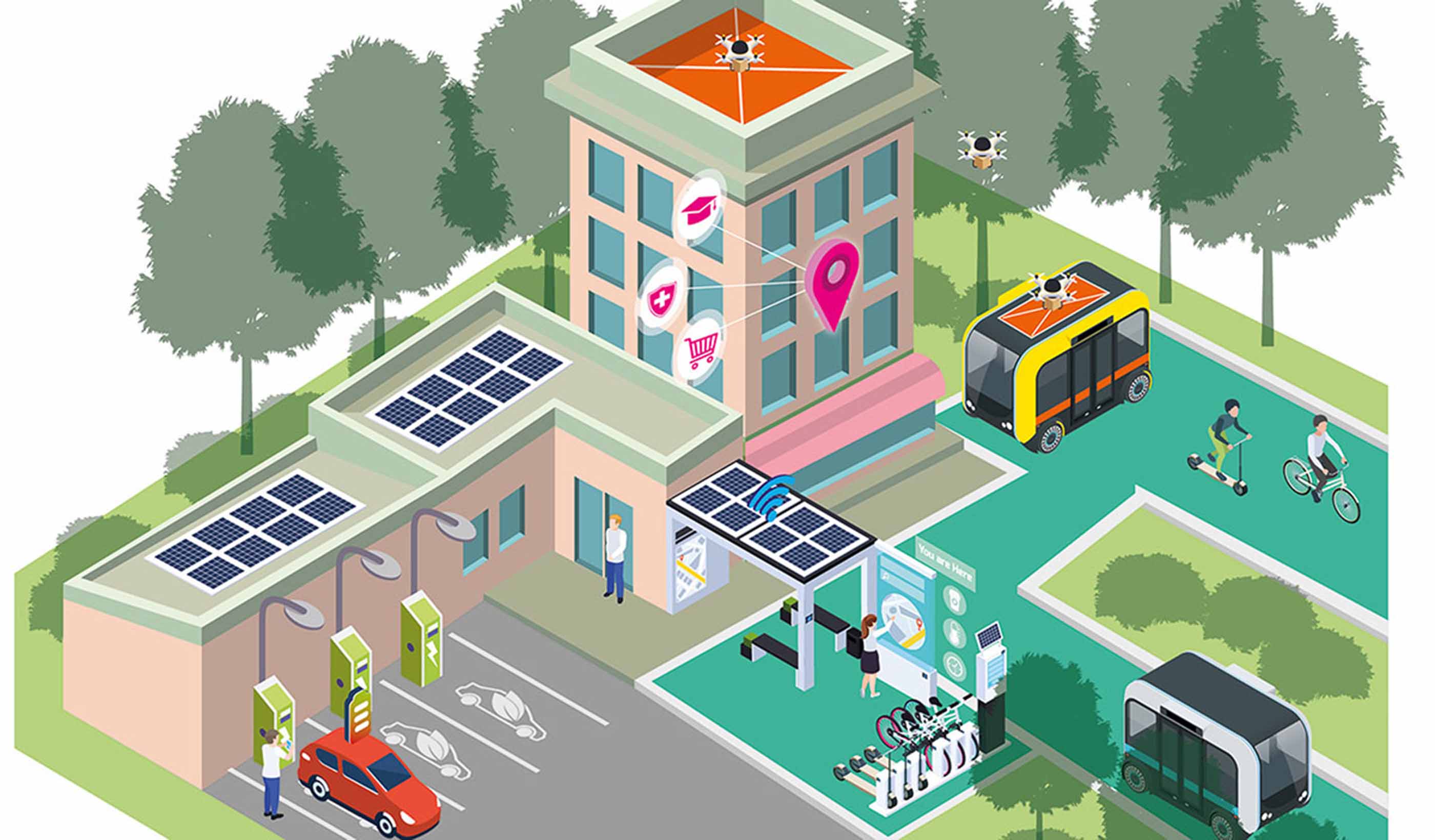
Blog Post How is transit responding to reopening?
-

Published Article Hydropower: A Cost-Effective Source of Energy for Hydrogen Production
-
Blog Post Certainty in uncertainty: Machine learning can improve engineering decisions
-

Video Design inspiration for creating spaces to inspire your students, researchers, and staff
-

Published Article Deliver smart buildings using CSI Division 25, commissioning
-

Blog Post Does your company understand the risks of not transitioning to a low-carbon economy?
-

Blog Post Best places to produce hydrogen? Look at a topographic map
-

Blog Post Reconsidering waste in mining
-

Blog Post Clean transport: Reducing carbon emissions on our roads
-

How do we power rural communities? By providing off-grid solutions
-

Blog Post Combating climate change across Australia
-

Blog Post Climate emergency: How governments around the globe are tackling the crisis
-

Published Article Vent tech shortage hits amid automation push
-

Webinar Recording Asset Integrity: Key considerations for blending hydrogen in your pipeline
-

Blog Post Mechanical engineering and industrial buildings: Why we need to go above and beyond
-

Blog Post Microtunneling 101: Good things come in small packages
-

Webinar Recording Demystifying Hydrogen Fueling for Transit Fleets
-

Blog Post Microtunneling: The next big thing
-

Whitepaper The nature of wind-forced upwelling and downwelling in Mackenzie Canyon, Beaufort Sea
-

Published Article Mining’s ESG stewards
-

Video For our US Audience: Deadly Engineering - Power Plant Catastrophes
-

Blog Post What will the future campus look like?
-

Blog Post 3 emerging trends that put the “community” in community rec center design
-

Published Article Water Power
-

Blog Post Holistic heroes: How lighting designers use efficiency and modeling to boost performance
-

Blog Post Capturing carbon: How nature-based solutions help achieve net zero goals
-

Blog Post Tackling climate change: Identifying global opportunities in response to the IPCC AR6
-

Webinar Recording ESG, Net Zero Emissions and Climate Change Resilience for Manufacturing
-

Blog Post Protecting Florida homes from invasive plant species
-

Blog Post How to overcome the 3 most common obstacles in long-term community development
-

Blog Post Continuous, holistic, and equitable health communities
-

Published Article Hydroelectric Dam Conversion with Diaphragm Walls, Retention Systems
-

Published Article Energy storage using conventional hydropower facilities
-

Webinar Recording What to consider when converting office or commercial space to science use
-

Published Article Giants of Design on Corporate Trends
-

Blog Post To incentivise or to tax, or both? How we can reduce carbon in our infrastructure schemes
-

Blog Post Fully aware: How to track air pollutants and GHG emissions with near real-time data
-

Blog Post Elevating mobility infrastructure into a destination of its own
-

Blog Post A call for a global carbon offset banding system
-

Published Article Top 100 Green Design Firms
-

Blog Post 5 things to know before starting an ITS deployment
-

Published Article Red Rock Hydroelectric Project: Successfully Generating New Power from a Pre-Existing Dam
-

Webinar Recording All Hands on Deck: Building Strong Public Partnerships for Your Next Water Infrastructure Project
-

Published Article Storage tunnel will improve Narragansett Bay water quality
-

Published Article New bridge inspection techniques increase speed, efficiency, and safety
-

Blog Post 6 factors when planning a school sports facility
-

Published Article Back in Business: Minimizing Spread of Disease Remains Priority
-

Blog Post How wastewater’s circular economy can help fight climate change
-

Published Article Adapting to climate change in New York City
-

Blog Post Helping mines leverage the “S” in ESG
-

Blog Post The pandemic helped some utilities boost resiliency and delivery—while reducing risk
-

Published Article Everything’s bigger in Texas: How a P3 mega roadway project came to life
-

Published Article Environmental Technologies to Treat Selenium Pollution: Principles and Engineering
-

Blog Post Passports to a net zero carbon future
-

Published Article Life Extension and Savings through Field Testing and Engineering Analysis of a Draft Tube
-

Published Article Waste-to-energy tech could slash carbon emissions, but its promise remains underdeveloped
-

Published Article UAE Plans to Burn Mountains of Trash After China Stops Importing Waste
-

Published Article How to step into the net zero zone
-

Blog Post How New Orleans’ response to Hurricane Katrina improved public safety and economic growth
-

Blog Post Listening to the land to design an urban Indigenous healthcare facility
-

Blog Post Innovative technology in the water sector: It’s time to focus on needs over preferences
-

Podcast Design Hive: Rebel Roberts and Paul Sereno on the Niger Heritage project
-

Published Article Planning ahead - the importance of waste planning within developments
-

Blog Post Saving bats and generating more power: Acoustic data is the key
-

Report COVID-19 Recovery Strategies for Downtowns
-

Published Article Big Ideas
-

Blog Post Following the flames: Preparing for landslides in wildfire country
-

Published Article Clean Slate
-

Blog Post Highlighting the hidden: Integrating operational infrastructure into the community
-

Published Article Graeme Masterton talks commuter systems, working from home
-

Blog Post What to consider when converting commercial or office space to life-science use
-

Published Article How Creative Designs Can Further a Vision of Sustainability and Resilience
-
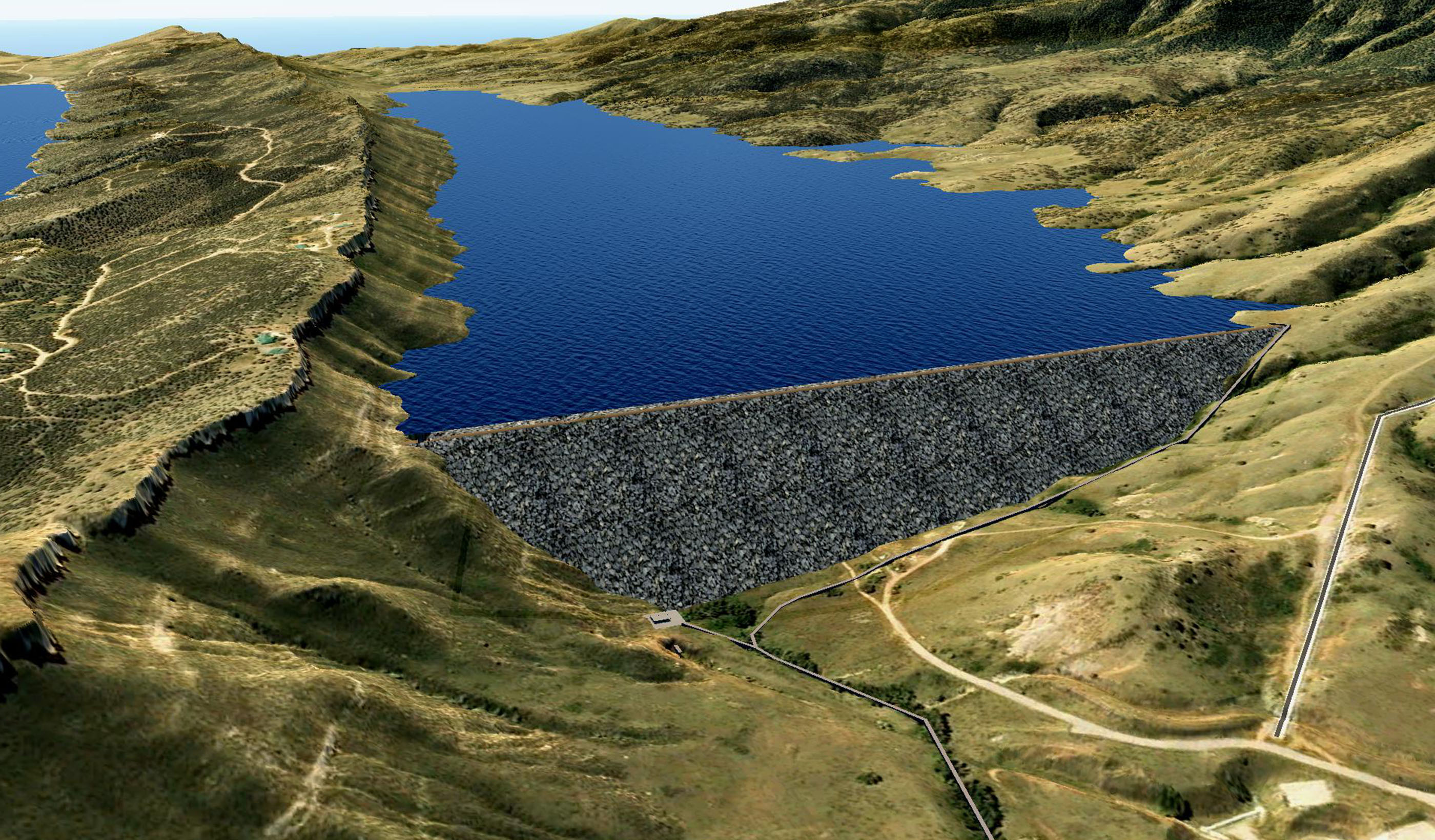
Published Article Chimney Hollow Reservoir Construction Risk Management
-

Webinar Recording Improving Dam Safety with Risk Informed Decision Making
-

Blog Post Elevating low-rise development—it’s a SWELL idea
-

Published Article Wellness in the Workplace: Burnout from the blurred lines of work and home
-
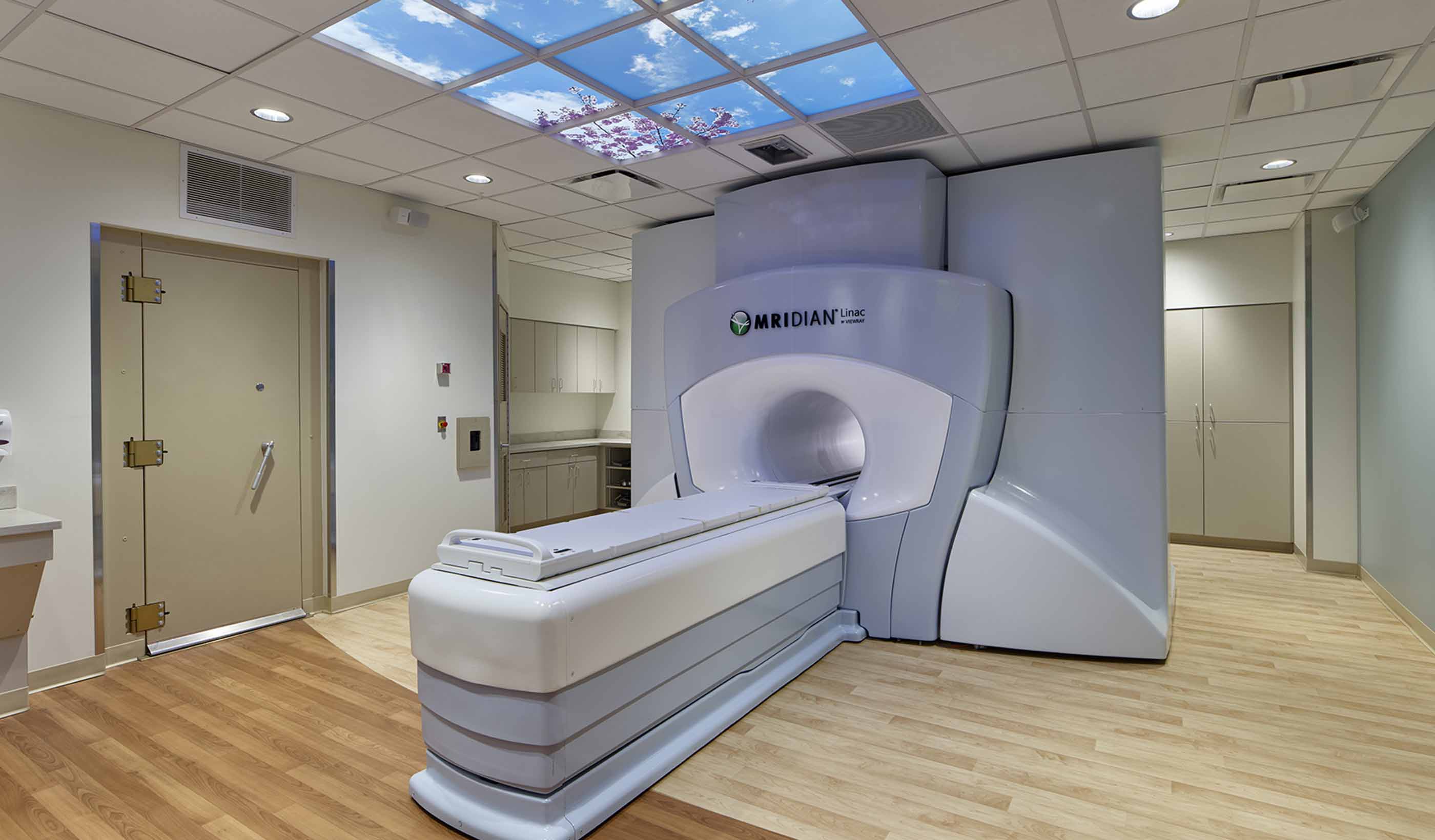
Blog Post Untapped potential: Expanding healthcare space, capabilities within a limited footprint
-

Published Article Airborne Electromagnetic Surveys: One More Tool in California’s Journey to Sustainable Groundwater Management
-

Published Article Can alternative tailings disposal become the norm in mining?
-

Publication Design Quarterly Issue 13 | After the Reset
-

Webinar Recording Adapting to 21st Century Water Resource Challenges
-

Webinar Recording Sustainable mining: The future is now
-

Blog Post Harmonizing the seen and unseen when designing for neurodiversity
-

Published Article Building a new paradigm: commissioning in the 21st century
-

Blog Post Don’t just offset carbon, remove it
-

Blog Post The port of the future: Secure, sustainable, and welcoming
-

Video Water policy institute pioneers solutions for sustainable water future
-

Blog Post Recycle your building: 8 reasons to consider adaptive reuse and retrofitting
-

Webinar Recording Designing with equity in mind: Rethinking how we approach projects
-

Blog Post Charged up: Key considerations around electric vehicle use on mine sites
-

Blog Post The potential of biogas in the energy transition
-

How can offshore wind power a more sustainable energy infrastructure?
-

What role should the energy industry play in a sustainable future?
-

Blog Post How can we reduce the carbon footprint of our oil and gas infrastructure?
-

Building partnerships for sustainable and resilient communities
-

Webinar Recording PFAS Investigations at Bulk Fuel Terminals and Refineries
-

Published Article 4 questions transit agencies should be asking about AVs right now
-

Presentation Designing for Impact: Hubs and Corridors
-

Blog Post The water industry is feeling the climate change squeeze
-

Published Article Top Trends in Tailings Closure
-

Article Micromobility Q&A: Are we on the road to a future of connected, Complete Streets?
-

Webinar Recording The State of the Art in Selenium Management and Regulation
-

Published Article 3,300-foot lifelines: Remote runway construction takes grit, group effort
-

Published Article From no natural light to low ceilings, interior designers share 15 clever fixes to design
-

Webinar Recording How to spend COVID relief funding to improve K12 environments
-

Published Article Not all carbon offsets are created equal
-

Blog Post Wastewater case study: 10 years. 4 procurement methods. 1 cutting-edge treatment project.
-

Video Providing Transportation Lifelines for Central Hawke’s Bay
-

Blog Post Community spirit can strengthen collective resilience—and you can design for it
-

Blog Post The eCommerce era of four-hour delivery
-

Blog Post More than just another bus route: Can Bus Rapid Transit spur compact urban development?
-

Video Creating a memorable passenger experience through art
-

Report What you need to know about the Coronavirus State and Local Fiscal Recovery Funds
-

Blog Post Plan B: Reasons to change your mining method from the obvious choice
-

Video Everyday essential resources
-

Blog Post As we return to the workplace, it’s important to ask: How safe is your building’s water?
-

Report Planning a resilient future for defense communities
-

Published Article The rise of water risk
-

Blog Post Can we learn to share? How the sharing economy can help launch the mobility revolution
-

Webinar Recording A New Way – Applying Asset Management to a Stormwater Program
-

Gull Bay First Nation Microgrid - Opportunities & Challenges
-

Webinar Recording Climate Change & Resiliency Planning
-

Blog Post Stantec Institute for Applied Science, Technology: Fueling innovation for utility systems
-

Webinar Recording Integrating Affordability Into Capital and Financial Planning
-

Blog Post Establishing clean water delivery is just the start: How to maintain investments long-term
-

Webinar Recording Lead in Drinking Water. Is Plumbosolvency an issue in New Zealand?
-

Published Article Emerging Trends in Sports Facility Design
-

Blog Post From zero to hero: Kick-starting the rebirth of North America’s malls
-

Blog Post We can think differently to create more equitable outcomes in city building
-

Blog Post Supporting equity with an inclusive neighborhood—it’s urban planning for all
-

Webinar Recording Using Customer Insight to Improve Operations and Asset Performance
-

Published Article Elevators and Fire Sprinklers: Fire Safety Moves On Up
-

Blog Post Designing Complete Streets for towns and suburbs, not just for big cities
-

Video Ottawa CSST: A life on the water and a personal project
-

Blog Post Celebrating Niger’s rich heritage with design that instills national pride
-

Blog Post Early education case study: Using design to nurture diverse learners
-

Report What do employees want in the office of the future? See what they told us.
-

Brochure Planning for a New Workplace Paradigm
-

Presentation Producing Hydrogen using Hydropower in the US
-

Webinar Recording Net Zero Emissions and Climate Change Resilience for Mining
-

Video Exploring the possibilities with 3D printing
-

Blog Post 6 steps for effective modularization and preassembly planning
-
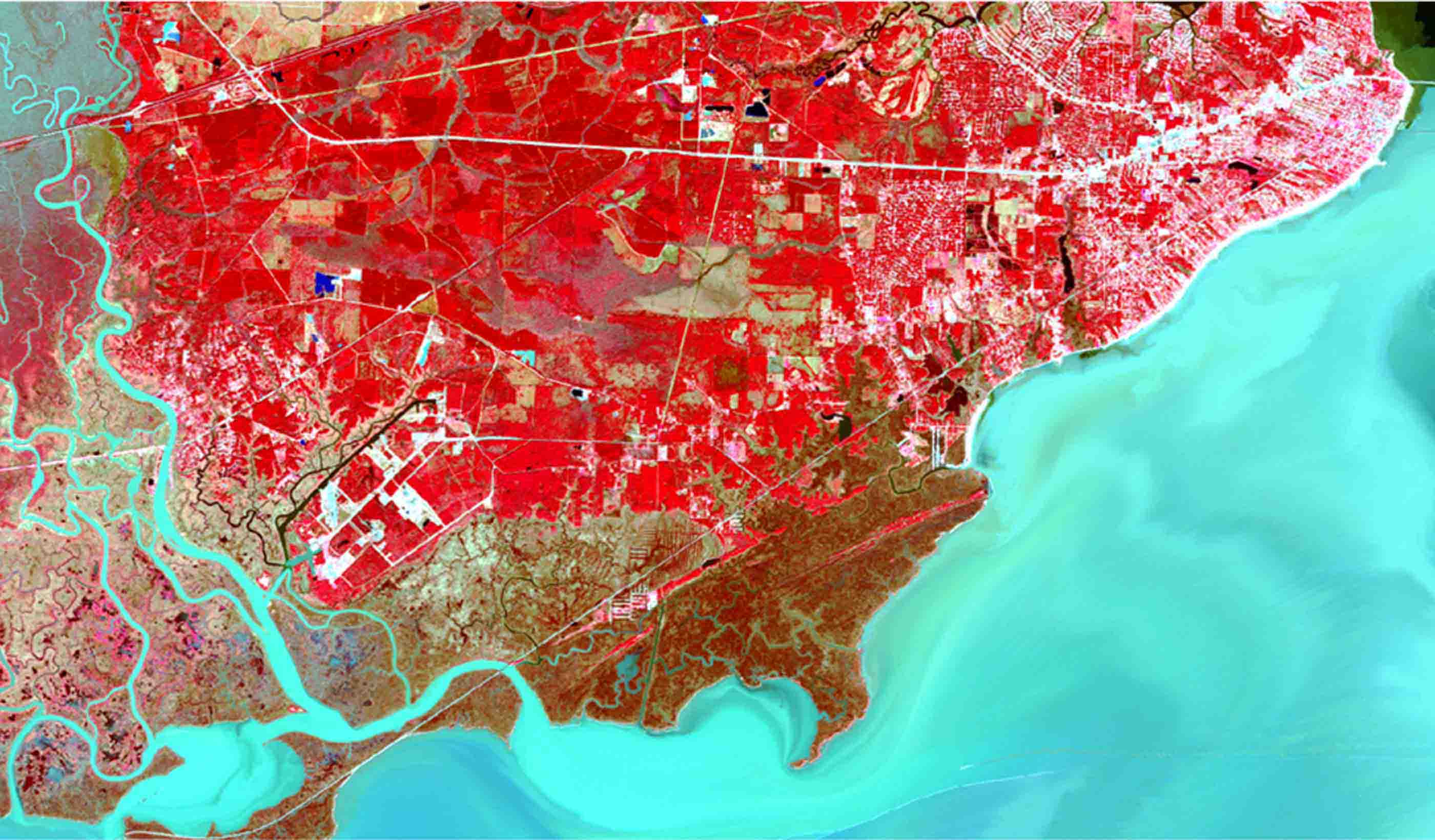
Blog Post How satellite image fusion and machine-learning can help us monitor large water bodies
-

Technical Paper A probabilistic model for assessing debris flow propagation at regional scale: a case study in Campania region, Italy
-

Publication Design Quarterly Issue 12 | Equity and Inclusion
-

Whitepaper What business are airports really in?
-

Published Article Living with Water in New Orleans
-

Published Article Why smart buildings are core to business continuity
-

Blog Post COVID-19 has accelerated the need for flexible labs and research environments
-

Article Taking design to new heights through market insights and innovation
-
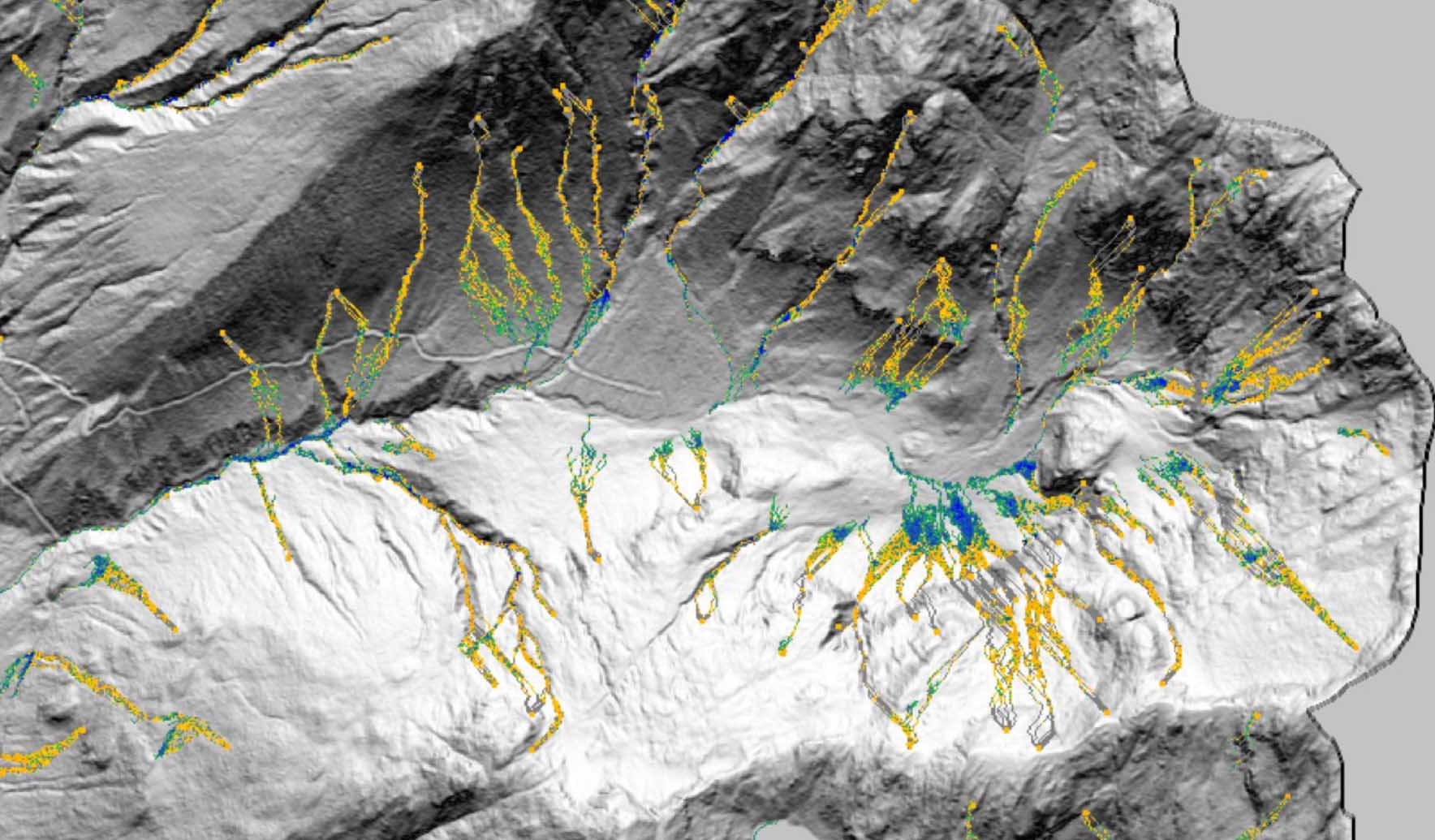
Technical Paper DebrisFlow Predictor: an agent-based runout program for shallow landslides
-

Podcast The Road to Autonomy
-

Published Article Office Design in a Post-Covid World
-

Blog Post 8 design strategies to enhance access to urban mental healthcare
-

Blog Post Clean energy case study: A conversation about powering Indigenous and remote communities
-

Video Rolling out new mobility pilots
-

Video Making mobility work for people
-

Video Planning for the future of mobility
-

Blog Post The American Rescue Plan Act: 4 questions to get thinking about infrastructure projects
-

Blog Post Finding success for downtown office space after COVID-19
-

Webinar Recording PFAS and the Energy Industry
-

Blog Post A new digital tool can help predict landslides, protecting people and infrastructure
-

Blog Post World Water Day: Valuing a finite, irreplaceable resource
-

Blog Post Beyond the terminals: How airports are helping strengthen communities and promote culture
-

Published Article Examining alternative project delivery
-

Blog Post Is digitization driving a new economic growth paradigm fueled by the circular economy?
-

Published Article Don’t damn ageing dams
-

Published Article Iowa Dam Adds Hydroelectric Power Generation
-

Published Article Double-edged sword for industrial sector
-

Podcast We need an EV charging renaissance
-

Published Article Engineers use hydropower from Red Rock Dam to generate clean and reliable electricity
-
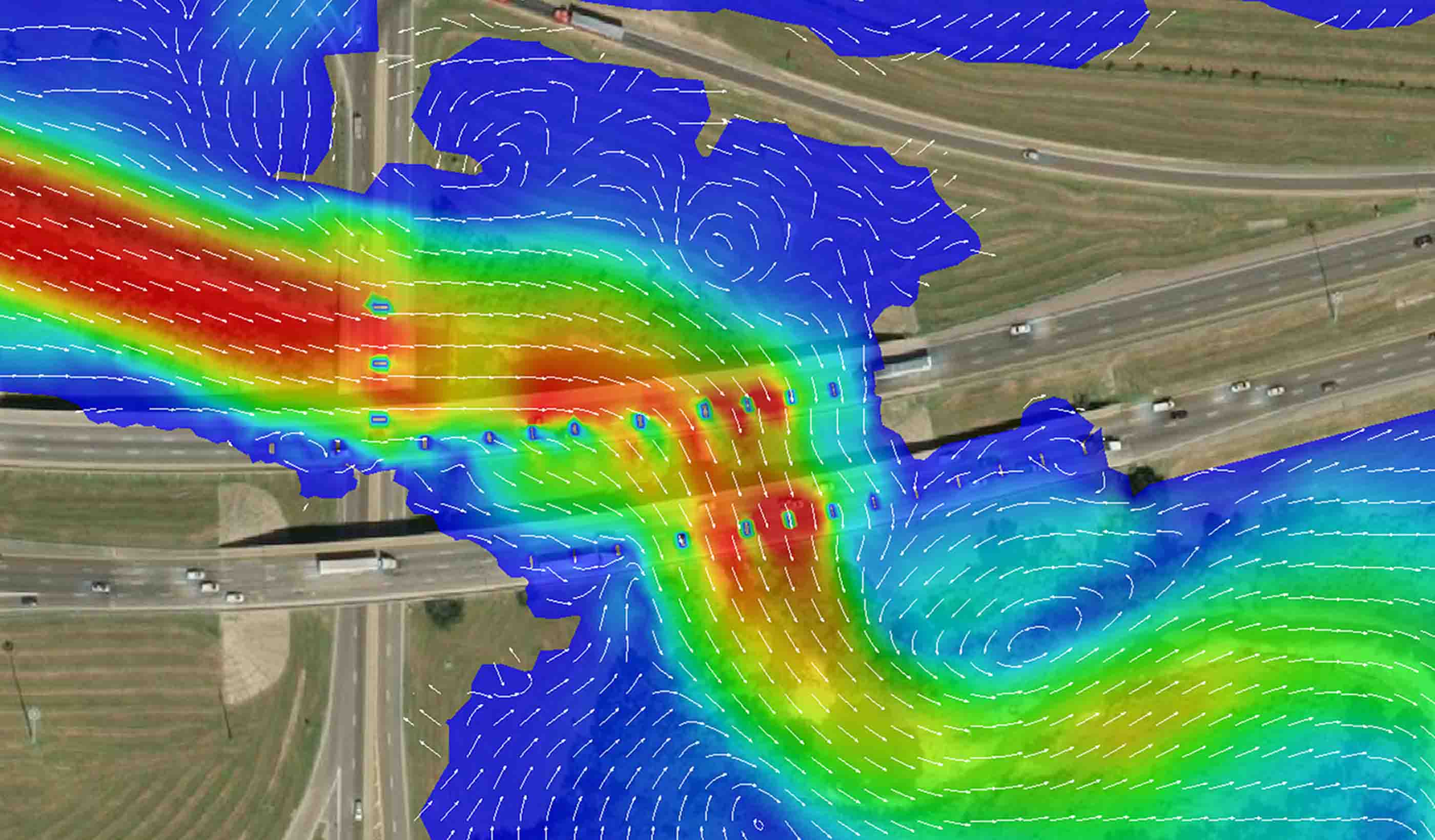
Blog Post Completing the picture: The future of hydraulic modeling is two dimensional
-

Published Article Chicago’s Historic Post Office Houses an Expansive Office for Walgreens
-
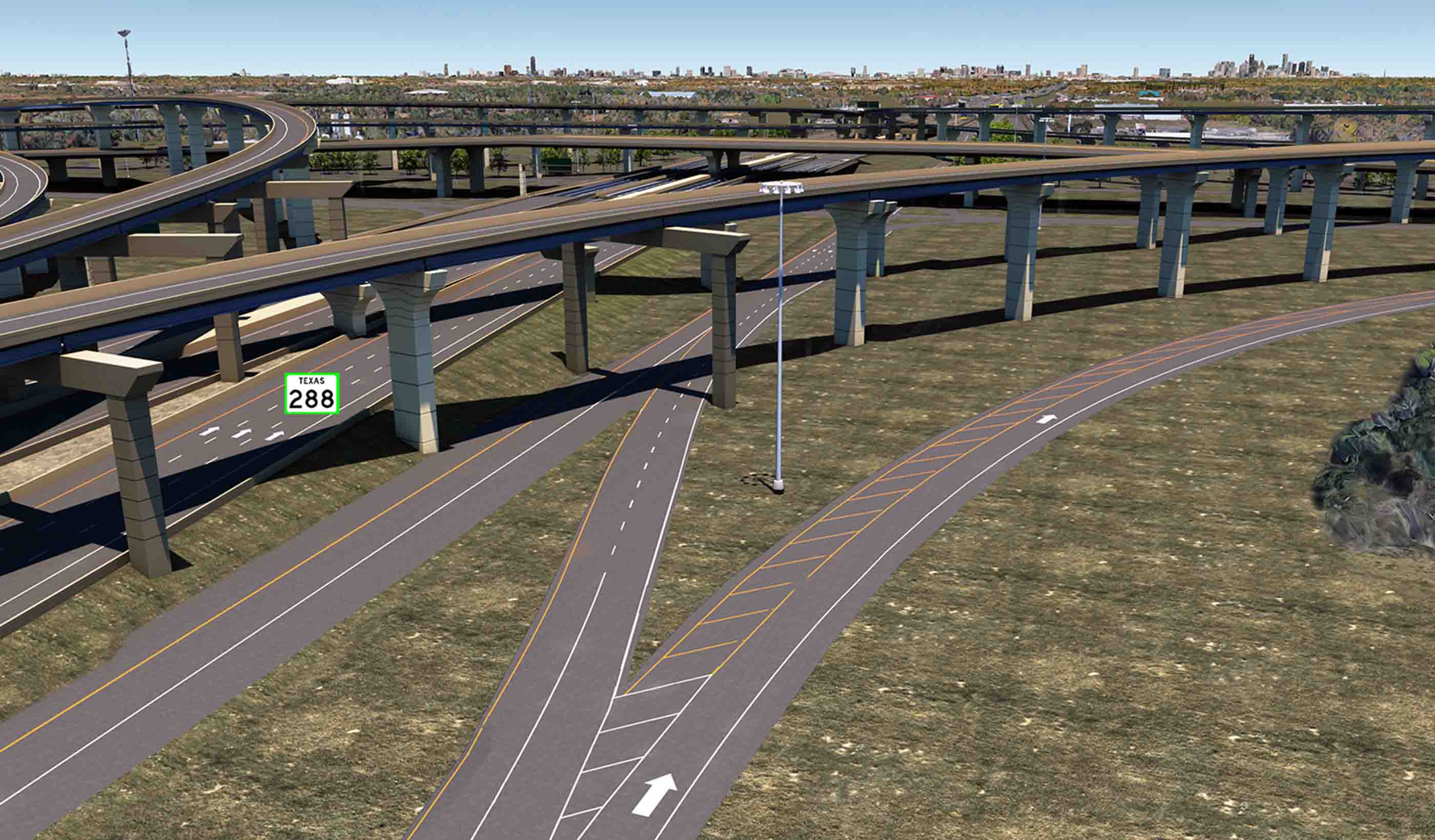
Blog Post 4 reasons why 3D modeling and BIM are the future of infrastructure design
-

Published Article Making Richmond Hill future-ready: Interview with David Dixon
-

Video Stantec kicks off the United Nations’ Decade on Ecosystem Restoration RIGHT!
-

Blog Post We can build better communities through landscape-led cities that focus on people not cars
-

Published Article Improving Children’s Wellbeing with Nature in Mind
-

Published Article Certification is Serious (but don’t just take my word for it)
-

Published Article How can strategic water resource options deliver best value for all?
-

Blog Post Keeping retail banks relevant (Part 2): Understanding generational preferences
-

Webinar Recording Integration of Company Culture and the Role of Engineering-of-Record
-

Blog Post Climate emergency: How communities are tackling the crisis
-

What role do buildings play in the energy transition?
-

Microgrids: A critical key to the energy transition
-

Blog Post How can hydrogen fuel a more sustainable energy future?
-

Blog Post The benefits of big hydro in Ethiopia
-

Video Global Leaders in Applied eDNA
-
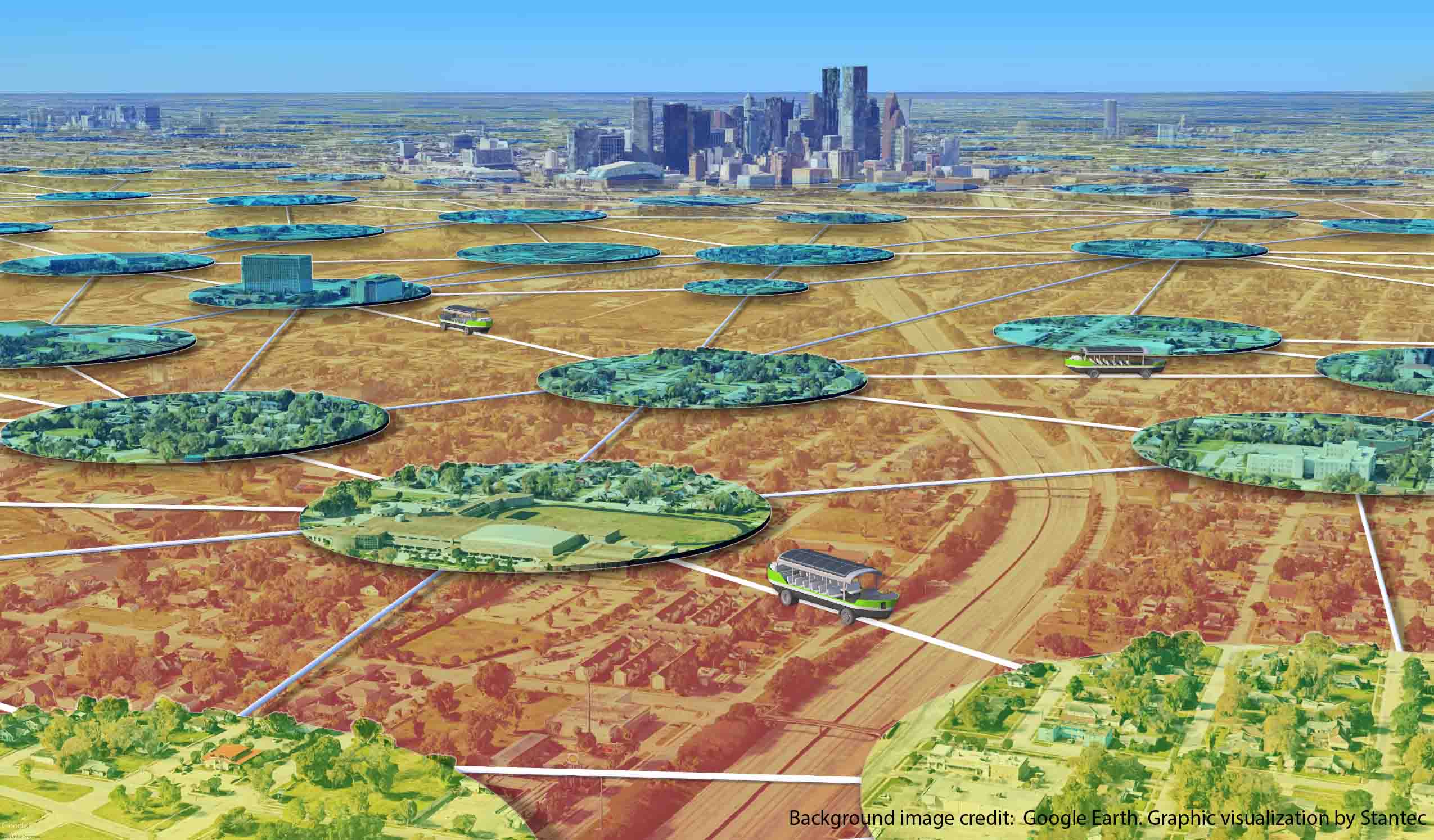
Blog Post The Lily Pad Network: A natural analogy to advance resiliency
-

Blog Post Creating adaptable AV policy to match AV technology
-

Published Article College Libraries Turn to Transformational Tech
-

Published Article Supplemental Grouting at Neelum-Jhelum Dam
-

Webinar Recording The Future of Resource Recovery
-

Blog Post Keeping retail banks relevant (Part 1): Becoming a post-pandemic “third place”
-

Published Article Think Tank: Stanley A. Milner Library Renewal
-

Blog Post Building water reliability: New Colorado dam, complex tunnel strengthens water supply
-

Blog Post Maintaining momentum: 3 ways to keep a large development project moving forward
-

Published Article Lights, candles, action: How St. John's can find more bright spots in the dead of winter
-

7 trends and techs—and 3 obstacles—shaping the big picture for an energy transition
-

Blog Post 3 public engagement practices for holistic urban planning
-

Publication Research + Benchmarking Issue 01 | How Buildings Shape Us
-

Webinar Recording Smart Utilities – Where is the World Heading?
-

Blog Post 3 important questions to ask when making a mining method change
-
Article A Stantec review of global AV research
-

Published Article The Nature Conservancy’s New Space by Stantec is Designed with A Colorado Nature Motif
-

Blog Post Accessibility isn’t just the law, it’s good business
-

Blog Post Wood and timber in student housing design
-

Published Article The State of the Global Dam Safety Practice
-

Published Article The Record Breakers!
-

Published Article Airports: Ensuring financial resilience through diversification
-

Blog Post Developing a new open space in your downtown community? Here are 5 key ideas to consider
-

Published Article Integrating a BAS into design
-

Blog Post 5 steps toward efficient building performance—from LEDs to heat pumps and more
-

Presentation Innovative design solutions for the future of micromobility
-

Webinar Recording Coagulation 101
-

Publication Design Quarterly Issue 11 | Empowering Design
-

Blog Post Sea change: 5 smart technologies to help us design better shipping gateways
-

Blog Post Building a legacy: Designing sports facilities that serve communities for decades
-

Webinar Recording PFAS: Regulations and Treatment
-

Whitepaper The Future of Mobility: Remaking Buffalo for the 21st Century
-

Published Article Deer Grove East: Restoring an historic forest preserve
-

Blog Post 3 challenges—and innovative solutions—when powering urban communities
-

Whitepaper Is your mine using remote sensing?
-
Webinar Recording Do more with Data - Digital Innovation for Integrity
-

Blog Post How to stave off the wintry chills and embrace the outdoors this COVID-19 winter
-

Published Article Hydroelectric Generators: The forgotten mechanical component
-

Published Article Advancing Cancer Care
-

Published Article 5 recommendations to cultivate digital-forward innovation in the built environment
-

Webinar Recording The Blue-Green Corridors Project in New Orleans
-

Published Article Innovative ideas to clear the air
-

Video Improving New Zealand housing
-

Webinar Recording Winter City Design
-

Blog Post 6 sustainable solutions for reducing GHG emissions at pipeline facilities
-

Webinar Recording Excess Soil – October 2020 Webinar
-

Blog Post Trail building 101: Exploring design details you may not have noticed on your local trail
-

Blog Post Hidden in history: Creating invisible mechanical systems in heritage buildings
-

Published Article Delivery with a difference
-

Blog Post Building industry diversity: 5 ways to empower disadvantaged business enterprise firms
-

Webinar Recording Assessing Nutrient Loading into Waterbodies from Reclaimed Water Practices
-

Blog Post Harnessing the power of mobile GIS apps to simplify field work and save time, money
-

Video Balancing impacts to wetlands with the need for critical infrastructure expansion
-

Blog Post What you need to know about Canada’s regulatory framework for GHG emissions
-

Blog Post Dinner in a “snow globe”—one designer’s plan to help restaurants during a COVID-19 winter
-

Blog Post How to turn shopping malls into a physical and virtual retail world
-

Webinar Recording Global Panel on COVID-19 Responses in Vulnerable Urban Contexts
-

Publication Water Futures +1 – Water Energy and Agriculture to 2035
-
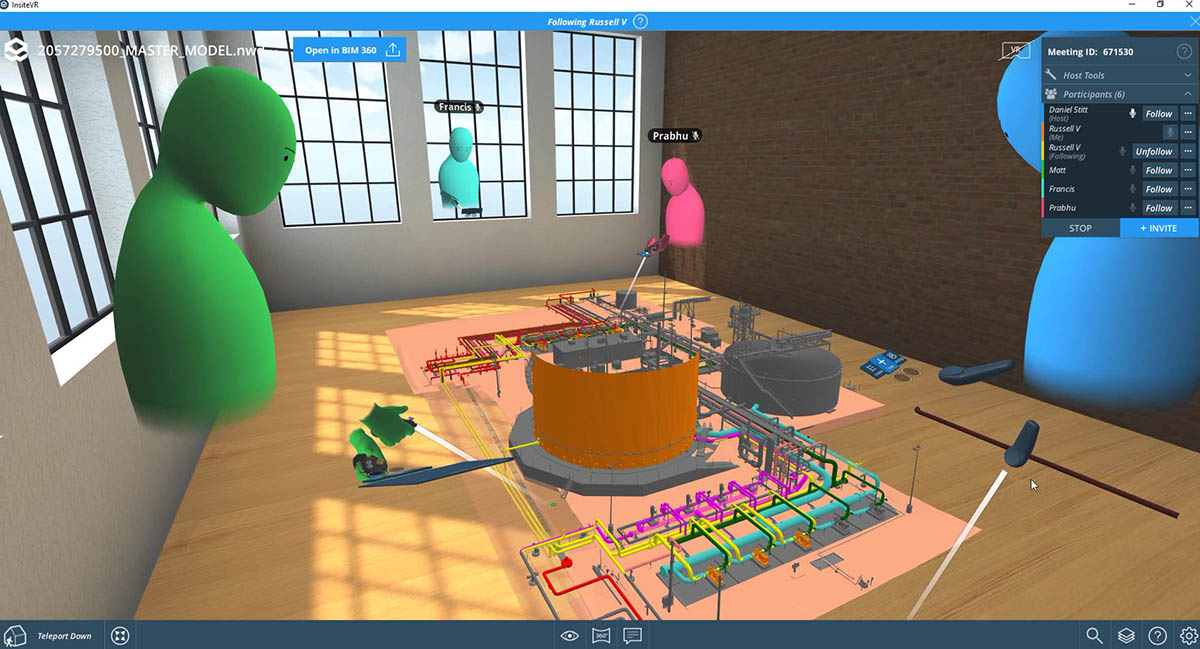
Published Article How virtual design changes the way we work
-

Electricity as a clean source of energy: Quebec’s example
-

Published Article Students, tech, COVID drive higher ed HVAC design
-

Published Article The Indigenous Hub in Toronto Promises a Brighter Future
-

Blog Post Working 9 to 5 has been a double climate change whammy
-

Webinar Recording Strategic Assessment on Climate Change
-

Published Article 5 reasons the Denver Metro area will be a post-pandemic “winner”
-

Published Article Something for Everyone at Riverlife Park
-
![[With Video] Untapped energy: Transforming existing dams into a source of hydropower](/content/dam/stantec/images/projects/0101/red-rock-hydroelectric-project-2.jpg)
Blog Post [With Video] Untapped energy: Transforming existing dams into a source of hydropower
-

Blog Post Carbon: A common language for change—now is the time to act
-
Published Article Mesocosm comparison of laboratory‐based and on‐site eDNA solutions
-

Blog Post Using digital solutions to capture, curate, protect, and analyze vital data
-

Webinar Recording Operations and Hazard Considerations in the Design of Treatment Plants
-

Published Article Pandemic Response Lessons From Singapore
-

Blog Post Warm welcome: What to consider when designing a modern mental health space
-

Blog Post How can digital data collection lead you and your project to success?
-

Published Article Future-proofing the industry
-

Webinar Recording Cerro Verde Mine Tailings Storage Facility
-

Published Article Renewal Rooms for Nurses
-

Published Article Taking down the walls around mental health care
-

Published Article Is this the beginning of a micromobility revolution?
-

Published Article The largest public charging station in the US showcases the challenges facing developers
-

Whitepaper How can Urban Agriculture Play a Role in the City of the Future?
-

Video Bridging communities while improving multimodal transportation
-

Blog Post Satellite technology provides a new way to assess historic pipeline releases
-

Published Article Tailoring Mental Health Design To Young Adult Patients
-

Webinar Recording Impacts of the Updated Steam Electric Power Generation ELGs
-
![[With Video] Why VR matters in healthcare design](/content/dam/stantec/images/projects/0053/ucsf-precision-cancer-medicine-bldg-1.jpg)
Blog Post [With Video] Why VR matters in healthcare design
-

Article The how and why of measuring your water footprint
-

Published Article Going viral: How the coronavirus pandemic could change the built environment
-

Published Article Core Considerations for Early Childhood Classroom Design
-

Webinar Recording Atlanta’s Regional Zero Waste Energy Recovery Program
-

Blog Post The pandemic’s lessons in loneliness and aging in place
-

Published Article Award-Winning Hydropower Project Helps Electrify Ethiopia
-

Blog Post Winter city design: 3 ways to save our small businesses this COVID-19 winter
-
Blog Post 5 critical factors to meeting deadlines for America’s Water Infrastructure Act of 2018
-

Published Article A global, standard approach to tailings, finally
-

Published Article Signaling a Safe Return
-

Published Article Basics of industrial, manufacturing facility design
-
![[With Video] Beyond the monument: Honoring our heroes through contextual design](/content/dam/stantec/images/projects/0094/boston-marathon-memorial-3.jpg)
Blog Post [With Video] Beyond the monument: Honoring our heroes through contextual design
-

Blog Post Making it easier to protect streams
-

Publication Design Quarterly Issue 10 | The Carbon Issue
-

Blog Post Continuing advances in wastewater-to-energy technology
-

Blog Post Creating a sustainable port for Canada’s Department of National Defence
-

Blog Post Remaining hands-on while distanced: How to take design-build collaboration virtual
-

Pipeline Threat Assessment for Safety Assurance
-

Published Article How This Veterans Research Building Evolved Amidst Design Changes
-

Blog Post The South Surrey Interceptor: A microtunneling milestone in North America
-
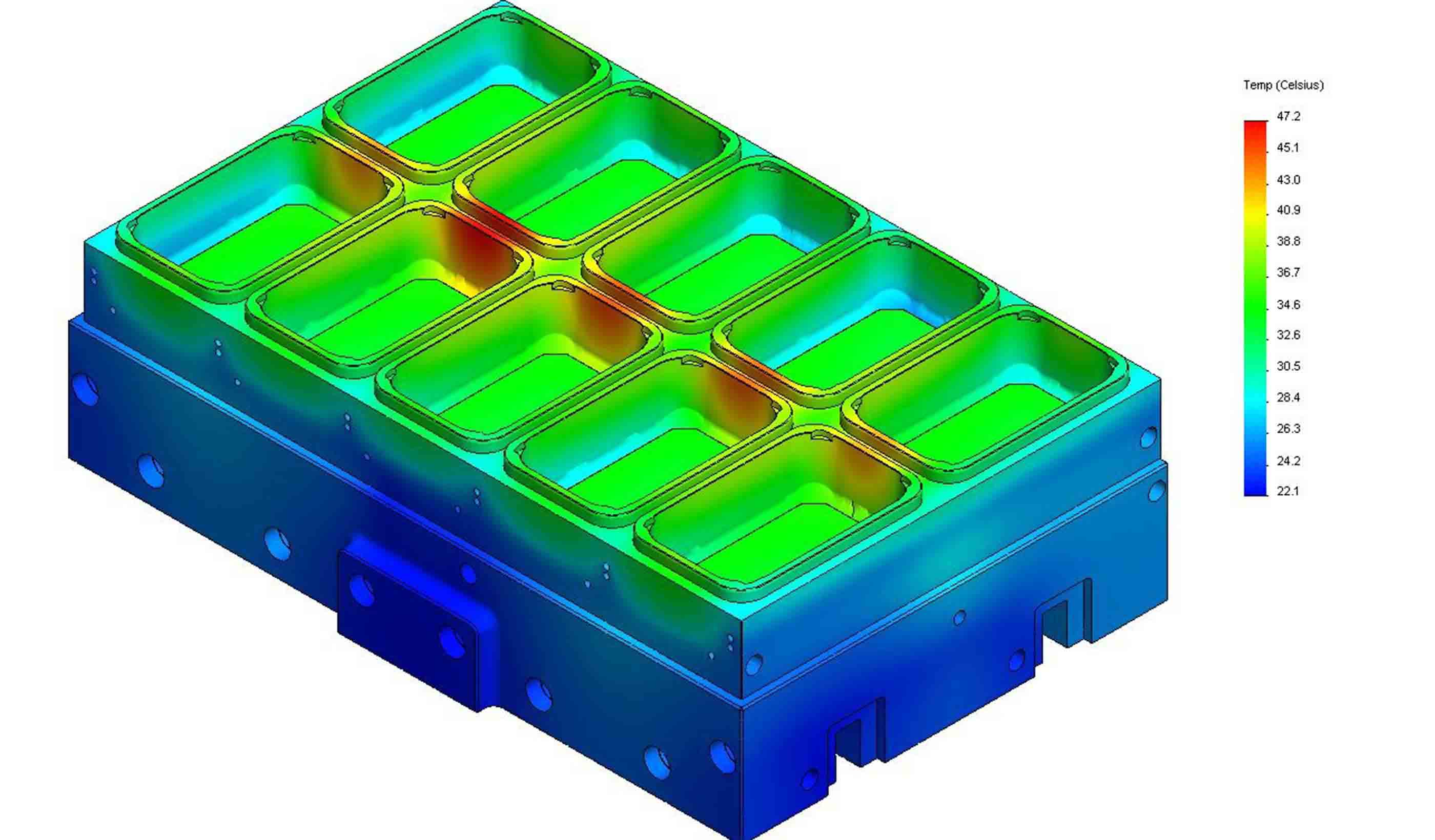
Blog Post What is Finite Element Analysis and how is it done?
-

Published Article A hybrid learning approach could redefine higher education
-
Published Article City of Somerville launches wastewater testing program to get ahead of COVID-19 spikes
-

Published Article How to make the most of COVID winter
-
![[With Video] How a young team shaped a new home for a mental health center](/content/dam/stantec/images/ideas/blogs/016/stellas-place-1.jpg)
Blog Post [With Video] How a young team shaped a new home for a mental health center
-

Blog Post 5 ways effective program management supports project owners
-

Blog Post Awaiting departure, 6 feet away: How will airport holdrooms adjust?
-

Published Article Acute Condition
-

Blog Post What does designing with the heart of healthcare mean for nurses?
-

Webinar Recording Mall Remix: breathing new life into old malls
-
Blog Post Did COVID-19 finally push us to completely paperless projects?
-

Published Article Stantec helps Ethiopia move towards ambitious power goals
-

Published Article Reconstruction could be COVID-19’s silver lining
-

Blog Post Beyond Complete Streets: Could COVID-19 help transform our roads into places for people?
-

Published Article Pandemic control in high-performance buildings
-

Blog Post How to focus on health and wellness in existing buildings
-

Published Article Denver Water: A Holistic Design For First-Of-Its-Kind Water Reuse Initiative By Stantec
-

Webinar Recording Just Suburbs: Creating Equitable Opportunities in Suburban Development
-

Blog Post Digital data collection for wildlife surveys leads to better—and faster—information
-

Published Article Room to Grow
-

Video Stantec welcomes you to the Decade on Ecosystem Restoration
-

Blog Post UV lighting in building infection control: Can passive light safely kill coronavirus?
-

Blog Post How has shopping changed over the past 100 years? A look at the evolution of retail
-

Blog Post 8 ways to accelerate your pipeline project
-

Published Article A glimpse of the future – opportunities to manage surface water
-

Brochure Getting Back to Productivity: Responding to COVID-19
-

Blog Post Deploying data to monitor aging pipelines
-

Whitepaper Data Management and Pipeline Integrity
-

Blog Post 5 strategies for creating safer, healthier hotel experiences
-

Published Article Going beyond climate change awareness
-

Blog Post How parametric design helps in the creation of custom interior elements
-

Blog Post Retail as a stage: Today’s extension of a centuries-old philosophy
-

Published Article Port of the Future
-

Blog Post From Stantec ERA: Updating and refurbishing our hydropower stations will pay off long-term
-

Blog Post Collaboration, cost savings, and quality control: The benefits of CM/GC project delivery
-

Blog Post Scanning smarter: Using reality capture on renovations
-

Blog Post Changes are coming to Oregon’s greenhouse gas reporting program—are you ready?
-

Blog Post Engineering our return to the workplace after COVID-19
-

Article Digital Impact Assessment: A Primer for Embracing Innovation and Digital Working
-

Blog Post Exploring the airborne transmission of the coronavirus and strategies for mitigating risk
-

Blog Post From Stantec ERA: Monitoring pipelines from space
-

Published Article Long Island Rail Road Expansion is a medley of moving parts
-

Blog Post Beyond sports: 4 ways investments in athletic facilities improve communities
-

Brochure Getting Back to School–K12: Responding to COVID-19
-

Blog Post Stantec survey: How will commuting patterns change due to COVID-19?
-

Blog Post (With Video) From Stantec ERA: Are aging power grids ready for the future?
-

Blog Post What will it take to get water quality and stormwater regulations to line up?
-
Published Article Published in STRIVE Magazine: Starting a journey toward homeless solutions
-

Brochure Looking to launch Automated Vehicles in your community? We can help.
-

Blog Post Building resiliency in transit design during COVID-19 and beyond
-

Blog Post Delivering clean, smart and inclusive growth: Rebooting the transport and places skills agenda
-

Blog Post From Stantec ERA: Old for the new - Investing in our past to create a clean energy future
-

Published Article Published in the Institute of Water Magazine: Tackling the Climate Emergency
-

Blog Post From the Design Quarterly: Hospitality design in the digital era. How “smart” is smart?
-

Blog Post 4 technologies for improving building sanitization in a post-pandemic society
-

Blog Post The many benefits of native grasses in your urban environment
-

Webinar Recording Understanding the opportunity and challenges of Hydrogen
-

Blog Post Reawakening theme park magic while staying socially distant and safe
-

Published Article Published in The Journal of Dam Safety: Personal Reflections on a Global Dam Safety Incident
-

Published Article Published in Mining Magazine: Culture of improvement
-

Brochure Supporting the Water Industry in a changing world
-

Published Article Published in World Water Magazine: Sustainability to resilience to action
-

Blog Post Bright places and gathering spaces: 5 tips to design a modern industrial learning center
-

Blog Post How can PFAS on a site affect insurance claims?
-

Blog Post Why COVID-19 should make transit rethink the fixed-route system
-

Blog Post Decoding Transportation Innovation: Exploring the Path to Automated Vehicles and Shared Mobility
-

Blog Post Living with water: Understanding flood risk in an era of climate variability
-

Blog Post Empathy, leadership, and listening during the pandemic pause
-

Published Article Published in ENR: Stantec-Jacobs to Design $1.9B Levee and Floodwall Along Texas Coast
-

Blog Post Restoring ecosystems in a post-pandemic world
-

Blog Post Can airports integrate health screening and security measures into a single process?
-

Stantec ERA Issue 03 | The Aging Infrastructure Issue
-

Blog Post If history is our guide, then COVID-19 will improve airport baggage check-in
-

Blog Post Looking at our streets differently now and after COVID-19
-

Published Article Why mobility modelling is the secret to return-to-work
-

Blog Post The 42nd Floor: A view into the space and technology of the workplace we will return to
-

Blog Post What is hydrogen’s place in the energy transition?
-

Published Article Published in Civil Engineering: Stantec-Jacobs to Design Gulf Coast Storm System
-

Published Article Warehouse, manufacturing facilities go high-tech
-

Published Article Infection control in office buildings: Preparing for re-occupancy amid the coronavirus
-

Blog Post How will energy demand change in the post-COVID-19 world?
-

Blog Post Using lighting IoT data to inform a safer office reentry strategy
-

Publication Design Quarterly Issue 09 | People, buildings & technology
-

Blog Post How emerging technology will make for safer, more efficient air travel
-

Blog Post Are medium-density neighborhoods the answer to community planning in the future?
-

Blog Post Pandemic lessons: Reliable internet is no longer a luxury—it’s a right
-

Blog Post Stopping algae in its tracks before it disrupts your water treatment plant
-

Publication Stantec Idea Hackathon: Toward a Smart Tampa (Idea Book)
-

Blog Post Temporary COVID-19 treatment centers: An Albertan approach to fighting the pandemic
-

Blog Post Airport traffic collapse—crisis or opportunity? Rethinking the business of airports
-

Blog Post Embracing the creative spirit: How the Tampa Idea Hackathon helped me to think differently
-

Blog Post Lasers to the rescue: Using technology to collect essential data during COVID-19
-

Blog Post COVID-19 is changing design paradigms
-

Webinar Recording Electric Futures
-

Blog Post A global view of design and urban planning post-COVID-19 (Part 4): How will cities change?
-

Blog Post A global view of design and urban planning post-COVID-19 (Part 3): New infrastructure
-

Blog Post 4 questions transit agencies need to ask themselves in the wake of COVID-19
-

Blog Post The transformation of engagement: Community considerations for driving online conversation
-

Published Article Published in Dezeen: "This is not a hospital" says architect behind McCormick conversion
-

Blog Post Planning for life in cities after the pandemic
-

Blog Post Extraordinary technology: Satellites can keep your project on track during the pandemic
-

Blog Post So, what happens when it’s time to get back to the office?
-

Blog Post A global view of design and urban planning post-COVID-19 (Part 2): Changing perspectives
-

Blog Post Water and climate change: Resiliency is key
-

Published Article Published in Work Design: Choosing Responsibly: Healthier Materials in the Workplace
-

Published Article Published: Seize the Moment and Shift the Dial
-

Blog Post An update from Italy: Supporting our clients during the COVID-19 Pandemic
-

Blog Post How will stimulus funding and post-COVID-19 recovery create a more resilient country?
-

Blog Post What’s in a number? Understanding reported PFAS concentrations
-

Blog Post A global view of design and urban planning post-COVID-19 (Part 1): Pandemic prevention
-

Article Bringing the Envision sustainable design framework to Europe
-

Blog Post Disaster preparedness in the midst of COVID-19
-

Published Article Published in Healthcare Design: Solutions for Pandemic-Related Healthcare Capacity Issues
-

Blog Post Resilience through diversity: Incorporating climate adaptation into wetland restoration
-

Blog Post Bats from scats: Using eDNA to safely identify protected bat species in a time of COVID-19
-

Blog Post The new “shovel ready”: Preparing for COVID-19 infrastructure stimulus funding
-
Blog Post How remote VR meetings help design teams overcome the challenges of COVID-19
-

Blog Post From the Design Quarterly: Looking back on the first large-scale net zero energy building
-

Article Why it’s time to reimagine long-term care facilities
-

Published Article Published in CIM Magazine: A deep dive into the history of mine development
-

Blog Post 5 ways to achieve a healthier work space now and after COVID-19
-

Blog Post The COVID-19 pandemic is proof that it’s time to implement virtual healthcare
-

Blog Post Managing our wastewater and its resources through biomimicry
-

Published Article Published in Alaska Public Media: New study on Arctic warming: Was 2017 a tipping point?
-

Published Article Published: Delivering compliance through effective environmental management
-
Blog Post Pandemic preparedness: How hospitals can adapt buildings to address worst-case scenarios
-

Published Article CLT rises to the challenge
-
Blog Post [With Video] Transitioning to recovery while preparing for a second wave of COVID-19 cases
-
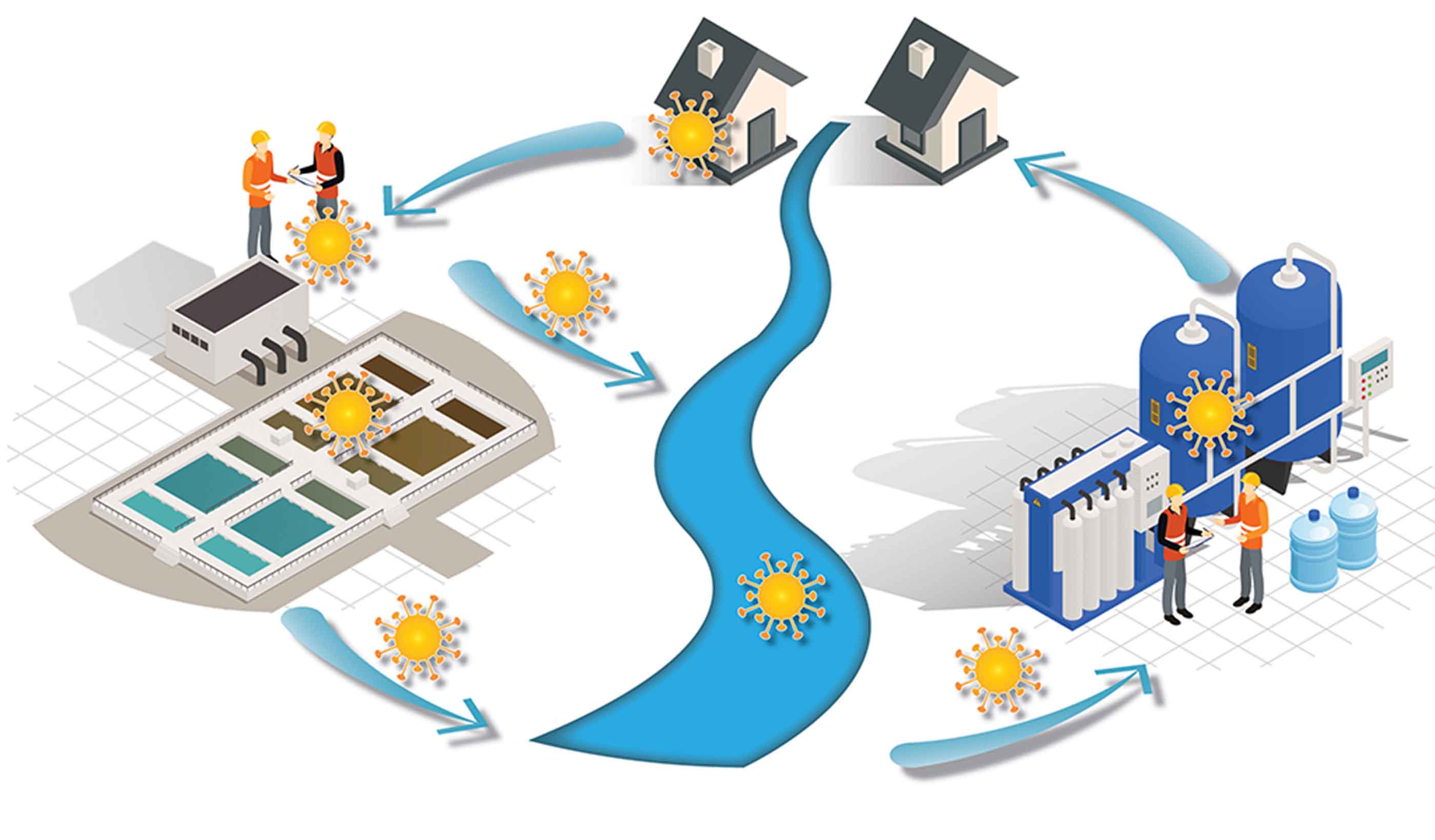
Webinar Recording Impact of COVID-19 on water/wastewater utilities
-

Webinar Recording The UK digital water utility experience
-

Blog Post It’s not if, but when: Designing healthcare spaces that support pandemic response
-

Published Article Published in Global Mining Review: Is Safety Suffering?
-

Published Article Published in Buildings: 5 Things to Consider Before Starting a Lighting Upgrade
-
![[With Video] From the Design Quarterly: How can lighting design influence wellness?](/content/dam/stantec/images/projects/0082/lincoln-square-bridges-136141.jpg)
Blog Post [With Video] From the Design Quarterly: How can lighting design influence wellness?
-

Blog Post Can drone technology help us mitigate the impact of future natural disasters? Absolutely
-

Blog Post How do office sounds impact design? Hint: Not all sound in the workplace is bad
-

Published Article Published in Retrofit: Designers Befriend a Researcher
-
Blog Post In the face of more extreme weather, how do we equitably fund stormwater utilities?
-

Blog Post The joys of recycling a building: How to get your adaptive reuse project on track
-
Blog Post Ontario’s new excess soils regulation: What do infrastructure owners need to know?
-
Blog Post Your guide to resilient infrastructure planning and investments
-

Blog Post From the Design Quarterly: What does it mean to fly well?
-

Blog Post Great lighting design—a key to an immersive experience and occupant well-being
-

Blog Post The time is now for risk-based water supply planning
-

Blog Post FAMS: Optimizing tough financial solutions for utility managers
-

Blog Post MDMER: What Canada’s revised mining effluent regulations could mean for your mine
-

Whitepaper Considerations for Water and Wastewater Treatment related to COVID-19
-
Blog Post Coronavirus and the water cycle—here is what treatment professionals need to know
-

Blog Post Celebrating the Year of the Nurse: Impacting hospital design as clinical consultants
-

Published Article Published in The Boston Globe: New high-rise in Chinatown will include affordable housing
-

Blog Post How technology is changing the patient experience—and what that means for designers
-

Blog Post 5 behaviors to stimulate sharing and collaboration for your team
-

Video Financial Analysis & Management System (FAMS)
-

Video Dubai takes another step towards Self-Driving Vehicles
-

Article CASE STUDY: St. Petersburg Stormwater User Fees
-

Blog Post From Stantec ERA: Changing field reporting through technology
-

Published Article Published: Rising to the challenge of delivering the largest ever UK national environmental programme
-

Published Article Published in Alaska Business: Architecture in Alaska
-

Blog Post Phytoremediation: What is it, and why might it be right for your contaminated site?
-

Published Article Published in Alaska Business: The art of architecture
-

Blog Post We need to start talking NOW about shared autonomous mobility—5 urban dividends
-

Video Orange County Coastal Storm Risk Management Project
-

Blog Post How advances in computer simulations can lead to more resilient coastlines
-

Blog Post From Stantec ERA: The era of virtual and augmented reality in the energy industry
-

Published Article Published in Environment Magazine: Building a resilient New Orleans
-

Blog Post Ontario’s new regulation for excess soil—what does it mean for you?
-

Blog Post Why start-to-finish construction management is imperative to a successful airport project
-

Published Article Published in Mining Magazine: Mine optimization through digital twinning and techno-economic modeling
-

Published Article Published: Thinking beyond the asset—a cost effective solution to the WINEP challenge
-

Blog Post Sponge city: Our urban landscapes have untapped potential to absorb stormwater
-

Blog Post From the Design Quarterly: Design drivers for a new behavioral and mental health facility
-

Blog Post Underwater remote sensing: Surveying the marine world safer, faster, and more affordably
-

Article Published: Cooperatives Offer Innovative Option to Solve the Affordable Housing Shortage
-

Blog Post The US Integrated Planning Bill: 10 steps municipalities can take to reap the benefits
-

Blog Post Friday Night Lights: The architecture of high school football stadium design in Texas
-

Blog Post A hospital that feels like home: Community as inspiration in healthcare design
-

Published Article Published in Waterbriefing: A new approach to water resource planning
-

Blog Post Does your city need a corridor study? Set the foundation for success with these 3 tips
-

Published Article Published in ASPIRE: Full-Service Engineering
-

Blog Post From Stantec ERA: 5 ways 3D modeling is changing the way we design power projects
-

Blog Post eDNA and metabarcoding—the future of effectively and rapidly surveying biodiversity
-

Blog Post Power through the storm: Improved style, function, and design for a more resilient grid
-

Blog Post Academic libraries of tomorrow: 4 models for storing your collections
-

Published Article Published in Waterbriefing: Helping the UK water sector deliver net zero carbon emissions
-

Blog Post From the Design Quarterly: How do you future-proof a stadium in the digital age?
-

Blog Post Combined sewage storage tunnels can help protect the environment and keep basements dry
-

Blog Post Waste not, want not: Turning human waste into a renewable-energy source for buildings
-

Published Article Published in Engineering Inc: Tunnel Visions
-

Whitepaper The housing white paper: a wish list
-

Article Published in Alaska Business: What about the water?
-

Publication Design Quarterly Issue 08 | Wellness & Active Lifestyles
-

Blog Post From the Design Quarterly: Meet me at the new mall—10 ways to make them cool
-

Blog Post Stantec’s Top 10 Ideas for 2019 (Plus 2 bonus items!)
-

Blog Post Ontario has updated its brownfield regulations—what do the 7 key changes mean?
-

Blog Post Feed the future: Human existence may depend on the success of the Second Green Revolution
-

Blog Post How satellite technology can monitor buried pipelines—and find leaks more quickly
-

Blog Post Better than before: 3 ways design can ease the transition to safety for survivors of abuse
-

Published Article Published in Community Design Collaborative: Carnell Scholars Drive Schoolyard Design
-

Blog Post From the Design Quarterly: Seeing civic infrastructure differently
-

Blog Post From the Design Quarterly: 6 approaches to waterfront revitalization
-

Published Article Published in International Mining: Bound for a Rebound
-

Video Thoughts from the American Water Summit - Sustainability
-

Video Thoughts from the American Water Summit - Innovation
-

Published Article Published in Global Water Intelligence: Coastal projects bring Stantec a flood of work
-

Blog Post Need a new marine crane? Follow these 7 guidelines for the procurement process
-

Blog Post 3 important keys to monitoring for stability during ash pond closures
-

Blog Post On the leading edge: Decentralized computing can transform smart-mobility infrastructure
-

Blog Post What’s cooking? When it comes to commercial kitchen design, it’s all about ventilation
-
Blog Post What to know about the proposed changes to steam electric effluent limitation guidelines
-

Blog Post Alternative project delivery is all about R&R—resourcefulness and repetition
-

Article Acid Rock Drainage: The Problem with Rates
-

Video Restoration and resilience post disaster
-

Blog Post Developing solutions to handle PFAS—aka “forever chemicals”—it’s an evolving science
-

Blog Post Want to elevate your smart city planning game? This 7-step roadmap gets you hyperconnected
-

Published Article Repurposing Retail Centers: Profiles in Adaptation, Repositioning and Redevelopment
-

Blog Post Research and benchmarking: Revisiting a 21st century library a decade after design
-

Publication Stantec ERA Issue 02 | The Digital Issue
-

Video What is a smart city?
-

Presentation Smart cities research: Building a Hyperconnected City
-

Article Avalanche safety offers lessons to prevent tailings dam failures
-

In California, 1 million residents lack clean water. Solutions are on the way.
-

Blog Post Redefining lost urban spaces: 5 ways to turn a laneway into a “lanescape”
-

Transport planning and the need to deliver more housing
-

Blog Post We are at the heart of the climate change challenge
-

Blog Post Global Asbestos Awareness Week
-

Blog Post From the Design Quarterly: 3 strategies to design academic research facilities to last
-

Blog Post How is our relationship with transport systems changing?
-

Blog Post Is the West Midlands Rail on track for economic growth?
-

Blog Post World Wetlands Day 2019
-

Blog Post Review of BEN Oxford to Cambridge Corridor Development Conference
-

Blog Post World Water Day 2019
-

Blog Post Standard method – help or hindrance?
-
Blog Post Discovering how “The Emily Test” can offer new—and better—perspectives to design
-

Article Published in METRO: 4 Factors to Consider For Zero-Emission Bus Fleet Transition
-

Blog Post 3 benefits to embracing an integrated approach to building design
-

Blog Post World Cities Day: Here’s how we can build a better urban life for future generations
-

Published Article Published in Mining Magazine: Mining Towards Renewable Energy
-

Blog Post New Zealand’s Three Waters services and the future of the water supply
-
Blog Post Next steps for artificial intelligence in the remote sensing digital evolution
-

Publication Community Futures: Think globally, act locally has never mattered more
-

Article Published in METRO: Enhance station safety, security by designing for transit user experience
-

Blog Post Consequence modelling: How to reduce the risk of an accidental release at your facility
-

Article Published in Railway Age: Transit-Oriented Development, Philadelphia Style
-

Blog Post 6 myths about embracing non-toxic materials in interior design
-

Blog Post From the Design Quarterly: House calls of the future
-

Article Published: Rebuilding Main Street in Rural America
-

Article Published in ARCHITECT: Integrating VR and AR Into Design Workflows
-

Article Published in Building Design + Construction: 2019 Multifamily Giants Report
-

Blog Post A spotlight on New Zealand's proposed National Environmental Standards for Freshwater
-

Blog Post A culture of convenience is finding its way to healthcare
-

Article Published in Water & Wastes Digest: Analyzing Combined Sewer Overflows
-

Blog Post What’s that car’s IQ?
-

Blog Post The hidden climate impact of the Internet of Things
-

Published Article Published: Reaching beyond stormwater to create resilient communities
-

Published Article Published in Logistics & Transport NZ: Using rail to take pressure off roads
-

Blog Post Creating communities through citizen science
-
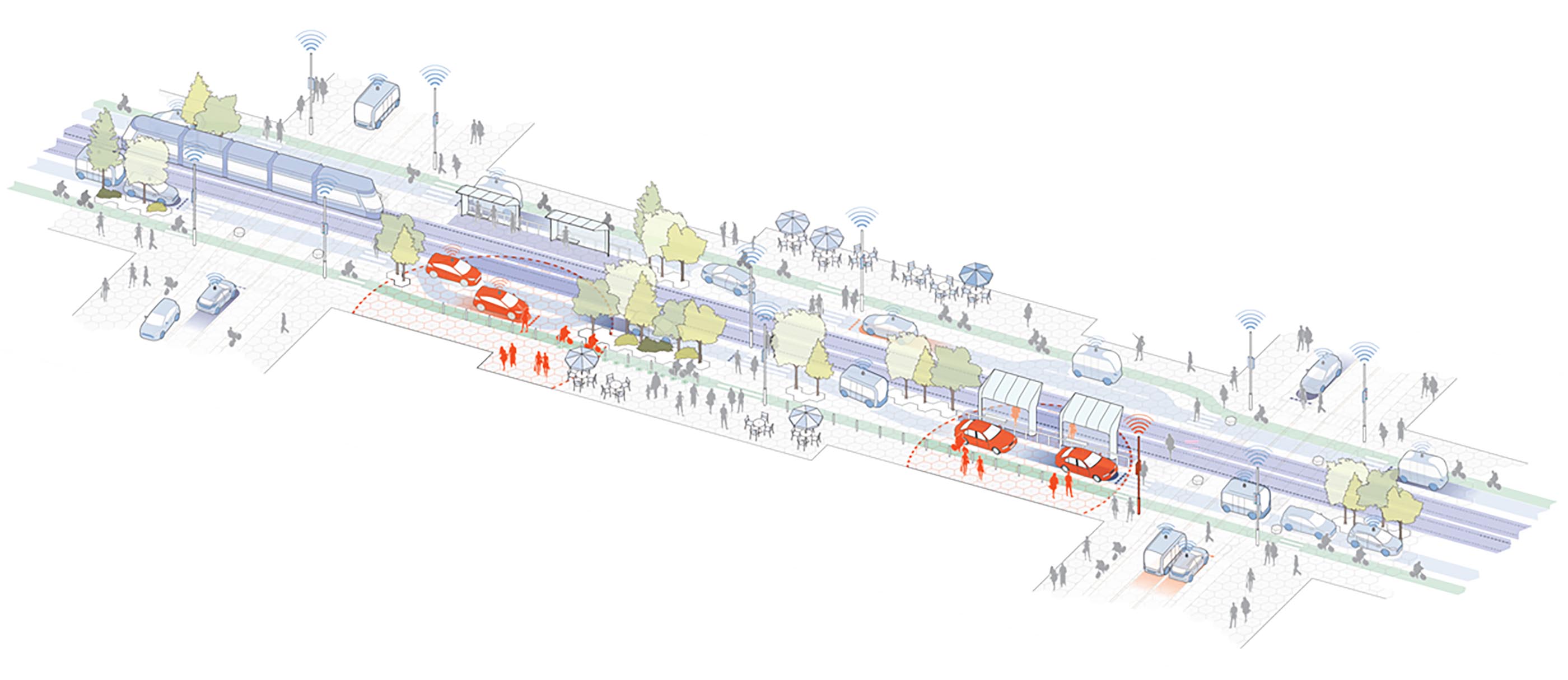
Blog Post Sidewalk Labs plan is setting a precedent in its approach to mobility
-

Published Article Published in How We Respond: New Orleans Residents Respond to Flooding w/ Citizen Science
-

Publication Design Quarterly Issue 07 | Adapting to Change
-
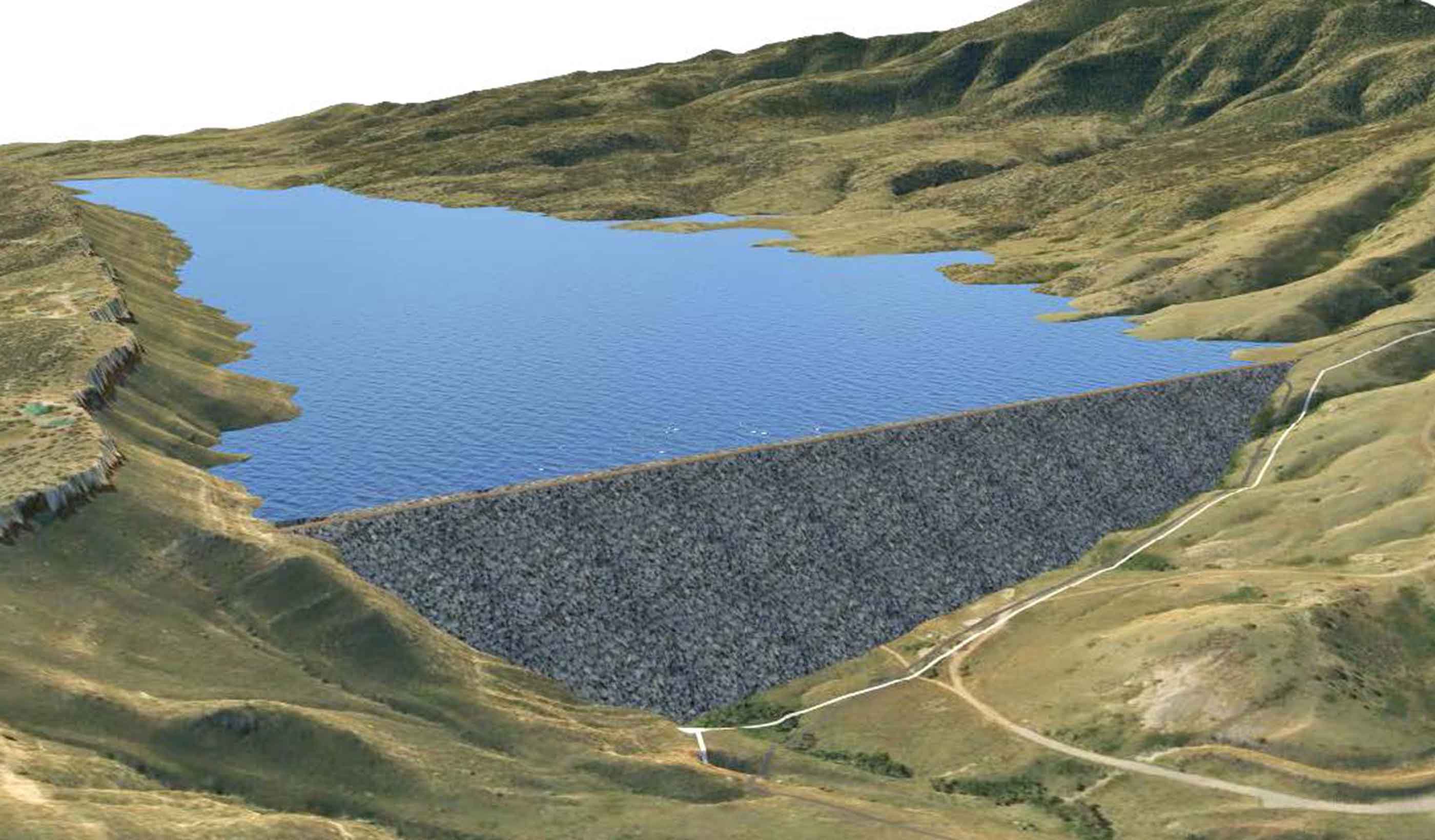
Published Article Published in International Journal of Hydropower & Dams: Complex tunneling at the Chimney Hollow dam
-

Blog Post The Internet of Environment: digitally twinning the planet
-
Blog Post New Zealand’s Three Waters Reforms: Prioritising the well-being of freshwater ecosystems
-

Article Published in Water Quality Products: The Sky is the Limit
-

Article Published in Medical Construction & Design: Grounded Art
-

Article Published in Medical Construction & Design: Sustainability and the Patient Experience
-

Blog Post From the Design Quarterly: Stantec's CEO explores how sustainability is at the core of our business
-

Article Published: Beat the Heat—4 Energy-Saving Design Tips for Hot Climates
-

Article Published: Cure for the Cold—4 Energy-Saving Tips for Buildings in Cold Climates
-

Article Published in PRISM: A healthy future for mass timber in medical facilities
-

Blog Post Mining and Indigenous communities—what matters most are relationships
-
Blog Post Become a conservation leader: The benefits of restoring pollinator habitat
-

Blog Post From the Design Quarterly: What are sustainability rankings and why do they matter?
-

Published Article Published in APWA Reporter: Water Resources Design for Long-Term Operations & Maintenance
-

Published Article Published in Providence Business News: Five Questions with Melissa Carter
-

Published Article Published in the Institute of Water: Delivering change through research-led innovation
-

Blog Post The connected, autonomous road ahead: Key challenges and opportunities for road operators
-

Published Article How cities can find funding for aging infrastructure
-

Article Published in Earth's Future: The Expanding Footprint of Rapid Arctic Change
-

Article Published in Canadian Consulting Engineer: Future City Planning
-

Article Published in Railway Age: Transit Oriented Development – Chicago
-

Published: Could Project 13 deliver a step change in efficiency for the UK water sector?
-
Blog Post How to bring clean energy to Indigenous and remote communities
-

Video Evolving Cities: Infrastructure Update
-

Presentation Presentation: #yearofthebus
-

Presentation Presentation: Transit Oriented Development: Holistic Value Capture
-

Webinar Recording What is Canada’s 1st accessibility assessment tool?
-

Blog Post A decade of ecosystem restoration: Protecting planet Earth for future generations
-

Published Article Published in Power: A Fine Couple They Are (Wind and Solar Power)
-

Blog Post Diverse industry partnerships strengthen community projects
-

Blog Post Virtual reality is the future of the oil and gas industry
-
Blog Post City planning: A guide to equitable planning for your community
-

April Vance – The sky is the limit at Stantec
-

Blog Post 3 ways to use water to cool your underground mine—affordably and sustainably
-

Article Published in ENR: Long-awaited Boston Marathon bombing markers reach the finish line
-

Article Recap of Stantec Presentations at the WCW Conference
-

Blog Post Weaving mental healthcare spaces into the neighborhood
-

Published Article Published in Western Canada Water: Choosing the right project delivery path
-

Published Article Published in NRU Toronto: Designing the smart city
-

Blog Post Hitting Saskatchewan carbon targets: How to achieve, measure, and prove reduced emissions
-

Blog Post From the Design Quarterly: 8 ways to design for density and live where the action is
-

Blog Post How Indigenous and northern communities shape my professional philosophy as a planner
-

Published Article Published in Planning magazine: May you live in interesting times
-
![[With Video] 3 surprising things I discovered from hacking 21st-century city challenges](/content/dam/stantec/images/ideas/blogs/002/with-video-3-surprising-1.jpg)
Blog Post [With Video] 3 surprising things I discovered from hacking 21st-century city challenges
-

Blog Post How can we optimize evolving lighting design to enhance health and well-being?
-

Video How do our library and learning commons designs stack up?
-

Published in World Water Magazine: Embracing change delivers SMART technologies
-

Blog Post Grounded art: Flooring in healthcare spaces increases in creativity and impact
-

Publication Stantec Idea Hackathon: Toward a Smart Toronto (Idea Book)
-

Article Published in Metro Magazine: Retrofitting garages for zero-emissions buses
-

Webinar Recording Saving our Suburbs (Part 3) – Smart Suburbs
-

Blog Post Building pride and community through high school athletics facility design
-

Blog Post How to use limited funding to reduce road fatalities and injuries
-

Published Article Published in Informed Infrastructure: Moving Connected and Autonomous Vehicles Forward
-

Blog Post 3 steps to deploying an autonomous vehicle shuttle pilot project
-

Published Article Published in Waterbriefing: What next for PR19?
-

Article Published in Alaska Business Monthly: Arctic infrastructure ingenuity
-
![[With Video] How can technology from video games bring flood risks to life?](/content/dam/stantec/images/ideas/blogs/001/with-video-how-can-1.jpg)
Blog Post [With Video] How can technology from video games bring flood risks to life?
-

Blog Post From Stantec ERA: The power of bringing hydropower and solar energy together
-

Blog Post Let it rain: A holistic plan for water use and reuse in a sustainable building
-

Blog Post From the Design Quarterly: Are net zero energy and net zero carbon buildings a must-have?
-

Published Article Published in IJHP&D: Upgrading the Salto Grande Scheme
-

Published Article Published in Hydro Review: Samoan Islands lead renewable energy efforts
-

Article Published in Railway Age: TOD: How New York got it right
-

Blog Post 3 techniques to boost community engagement for challenging mining projects
-

Article Published in Railway Age: P3s key for Transit Oriented Development
-

Blog Post Healthcare design choices: Is polyurethane upholstery fabric the best option in hospitals?
-

Blog Post From Stantec ERA: The future of the mining industry in a renewable-energy world
-

Published Article Published in International Mining Magazine: A Breath of Fresh Air
-

Published Article An in-depth look at the new leader of the Delta Stewardship Council as published in The Water Report
-
![[With Video] Envisioning the revitalized design of the Old Sacramento urban riverfront](/content/dam/stantec/images/ideas/blogs/004/old-sacramento-waterfront-1.jpg)
Blog Post [With Video] Envisioning the revitalized design of the Old Sacramento urban riverfront
-

Blog Post Elderly care: Can design cure loneliness as we focus on aging in place?
-

Publication Design Quarterly Issue 06 | Destination Zero
-

Blog Post From the Design Quarterly: Embracing the unknown when thinking big data and smart cities
-

Presentation Presentation: Water Reuse: Drivers and Successes
-

Published Article Published in Alaska Business Monthly: Top-flight priorities
-

Blog Post From Stantec ERA: 5 reasons why oil companies are investing in solar energy
-

Published Article Published: Minimal Disturbance: Milestone Project Tests Capabilities of Microtunnelling
-

Article Building on our community roots to help North American orchid conservation
-

Blog Post How can we build smart cities of the future?
-

Published Article Published in Engineering & Mining: Changing the Face of Mine Ventilation
-

Blog Post From the Design Quarterly: How are we designing for livable cities today?
-

Blog Post From Stantec ERA: What are the options for Alberta’s oil sands in a low-carbon future?
-

Blog Post Designing wellness communities to focus on integrated healthcare—reducing costs naturally
-
Published Article Published on WWT Online: The robots aren't coming... they’re already here
-

Blog Post How to navigate aging infrastructure and utility conflicts in urban areas
-

Published Article Published in American Infrastructure: The AI Interview – Lisa Beutler, President of the AWRA
-

Video Greenlight on Orchids
-

Published Article Why design review panels deliver
-

Published Article Published in Railway Age: Innovation From a ‘P2’ System
-

From Stantec ERA: Pumped storage is the answer to the sustainable energy-storage dilemma
-

Article Published in NC Currents: Storm Damage Risk Reduction – Permanent Canal Closures and Pumps
-

Article Predicting the unpredictable: How a Stantec toolbox helps us understand future flood risks
-

Blog Post Why the most effective school designs go beyond safety and security
-

Blog Post From the Design Quarterly: Technologies and trends changing how we power our world
-

Published Article Published in Planning Magazine: Curb control
-

Blog Post 3 steps dam owners and communities should commit to today to address dam safety
-

Published Article Published in Mining Magazine: Making the most of EPCM partnership
-

From Stantec ERA: Offshore wind energy is the green future of electricity generation
-

Video A Day in the Life of Rajesh
-

Blog Post 4 design features of a modern medical office building that make it a community landmark
-

Article Moxy Hotel redevelopment provides window to the past
-

Blog Post Infrastructure Week 2019: Parks as infrastructure—providing resilience and quality of life
-

Blog Post Infrastructure Week 2019: Brownfield development is the future of the US urban landscape
-

The power of mentorship: Choosing to help students find their career path
-

Blog Post Infrastructure Week 2019: The future of green infrastructure
-

Published Article Published in CIM Magazine: Front-end strategies to reduce project risks
-

Published Article Published in Mining Magazine: Consulting with the consultants
-

Published Article Published in Green Operations: Holistic Healthcare
-

Blog Post America is running out of engineers – here’s how we start recruiting more
-
Blog Post Illuminating trends for better healthcare
-

Blog Post Wet-weather infrastructure: Smart analysis and TOTEX planning equal savings and resilience
-

Published Article Published in REDNews: How updates to floodplain mapping will impact Texas
-

Blog Post From the Design Quarterly: Housing design that keeps the city within reach
-

Stantec ERA Issue 01 | The Energy Remix
-

Pitch Perfect
-

Published Article Published in Water & Wastes Digest: Reaching Deep Tunnel Vision
-

Blog Post A path to improving national standards for stormwater practices
-
Blog Post [With Video] 5 ways to design a transit maintenance facility that’s also a people place
-

Published Article Published in ENR: Drilling down to defend against drought in Atlanta
-

Published Article Published in WET News: In Focus - Social value and procurement
-

Published Article Published in Infrastructure Intelligence: The Project 13 initiative—engineering behavioural change
-

Blog Post Strapped for cash: How to help fund public projects for communities
-

Video All Things Urban: Joe Geller talks Stantec’s Urban Places
-

Published Article Published on Mining.com: Five steps to take today to make the most of your drilling program
-

Subsurface Utility Engineering enhanced through collaboration
-

Published Article Resiliency or sustainability: Which is most important when designing for transit agencies?
-
Blog Post This Earth Day, let’s embrace the full spectrum of sustainability
-
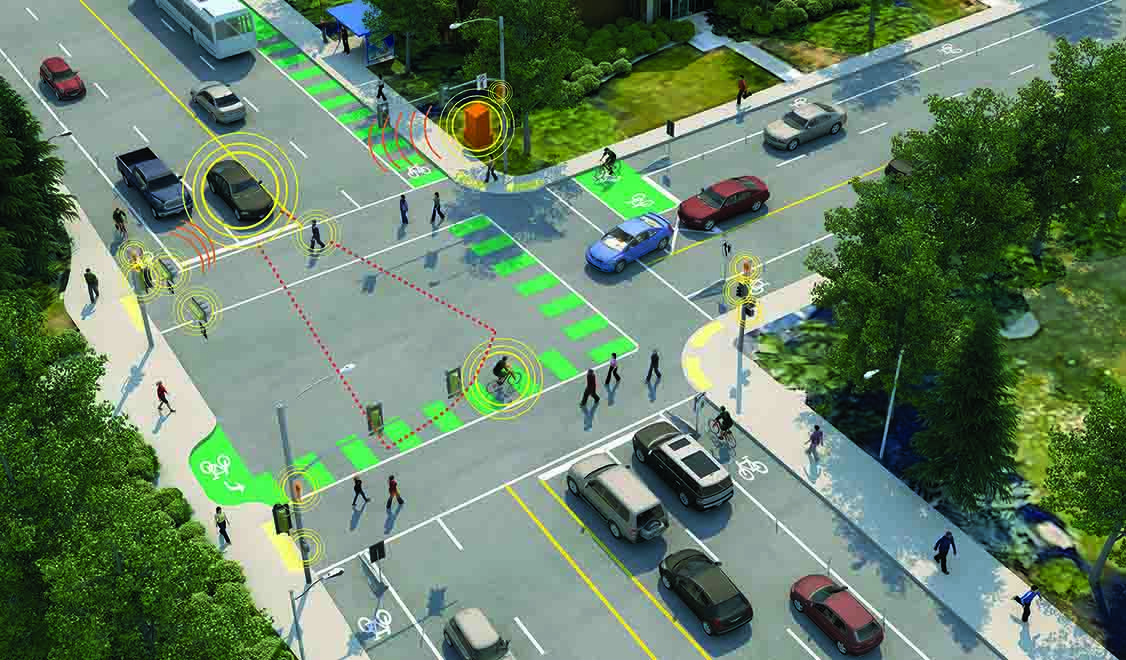
Blog Post How to plan for the future of electric vehicles
-
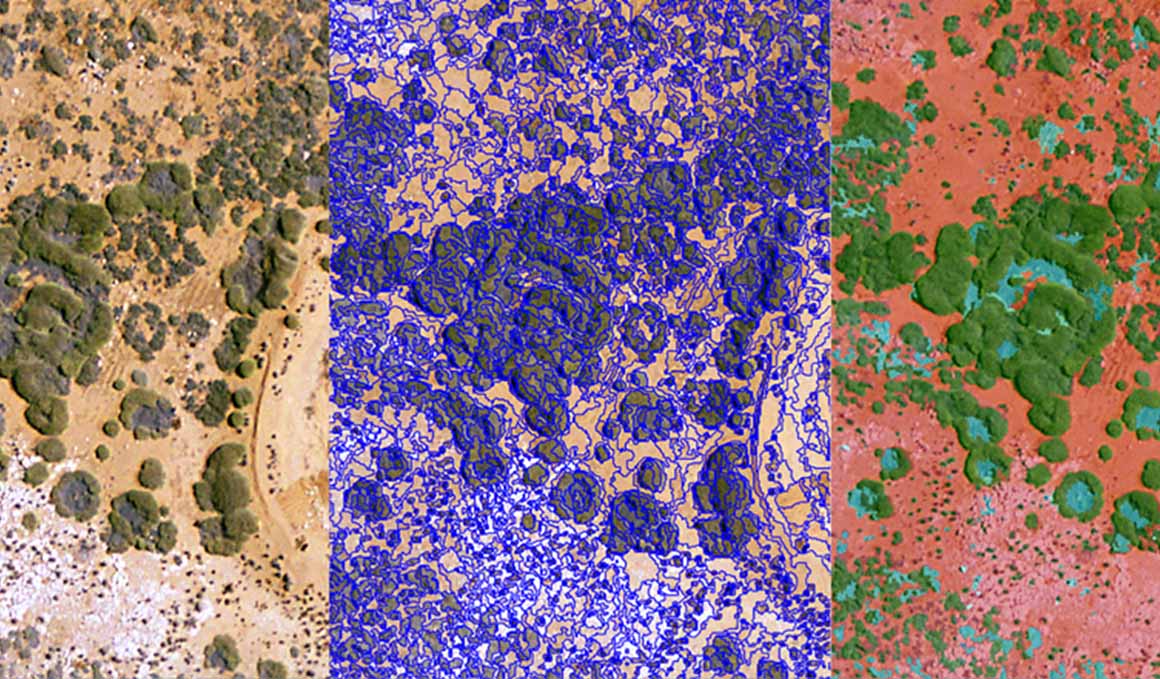
Blog Post The trickle-down effect of enhancing satellite technology
-

Published Article Published in The Institute of Water Magazine: Developing with our water environment
-

Published Article Published in CIWEM Environment Magazine: Creating a flood-resilient future for Yorkshire
-

Blog Post From the Design Quarterly: Embracing modular and prefab for future-ready design
-
Blog Post Back to our (grass)roots: New guide aims to decode grass and rush plant families
-

Blog Post Brick-and-mortar retail is not dead—here’s proof
-
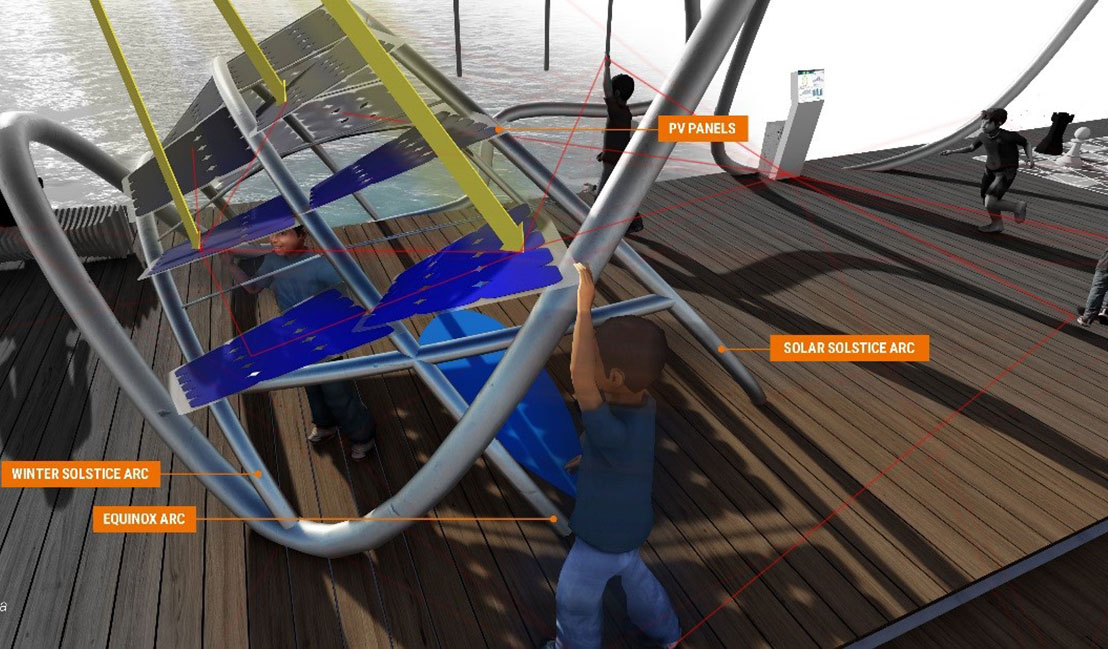
Blog Post From the Design Quarterly: Teaching sustainability and energy conservation through play
-
Blog Post How can we improve access to clean drinking water and sanitation around the world?
-

Blog Post Energy efficiency is really about energy productivity—and that’s about the bottom line
-

Blog Post Crazy airport runways: Landing on half a runway—why it’s business as usual in Alaska
-

Published Article Published: Four considerations before embarking on a carbon therapy center
-

Published Article Published in Water e-Journal: Building a Water Resilient Town
-

Video Natural Channel Design Methods: Better Together
-

Blog Post 6 ways to brand your property
-

Design Quarterly Issue 05 | Smart & Livable Cities
-

Published Article Published in IWP&DC Magazine: Top 10 Memorable Projects
-

Blog Post Art and architecture meet in the desert
-

Published Article Published in Waterbriefing: Innovation and integration will help prevent global water crises
-

Blog Post What happens downtown doesn’t stay downtown: The ripple effects of a strong center city
-
Blog Post Downtown Columbus, Ohio: A case study in the mobility revolution
-
Published Article You thought diversity was difficult?
-

Article Forming unique alliances to help Seychelles adapt to a changing climate
-

Blog Post Micro-apartments: Are these tiny units coming to the suburbs?
-

Blog Post Self-service bag drops and the challenges of speeding up airport baggage check-in
-
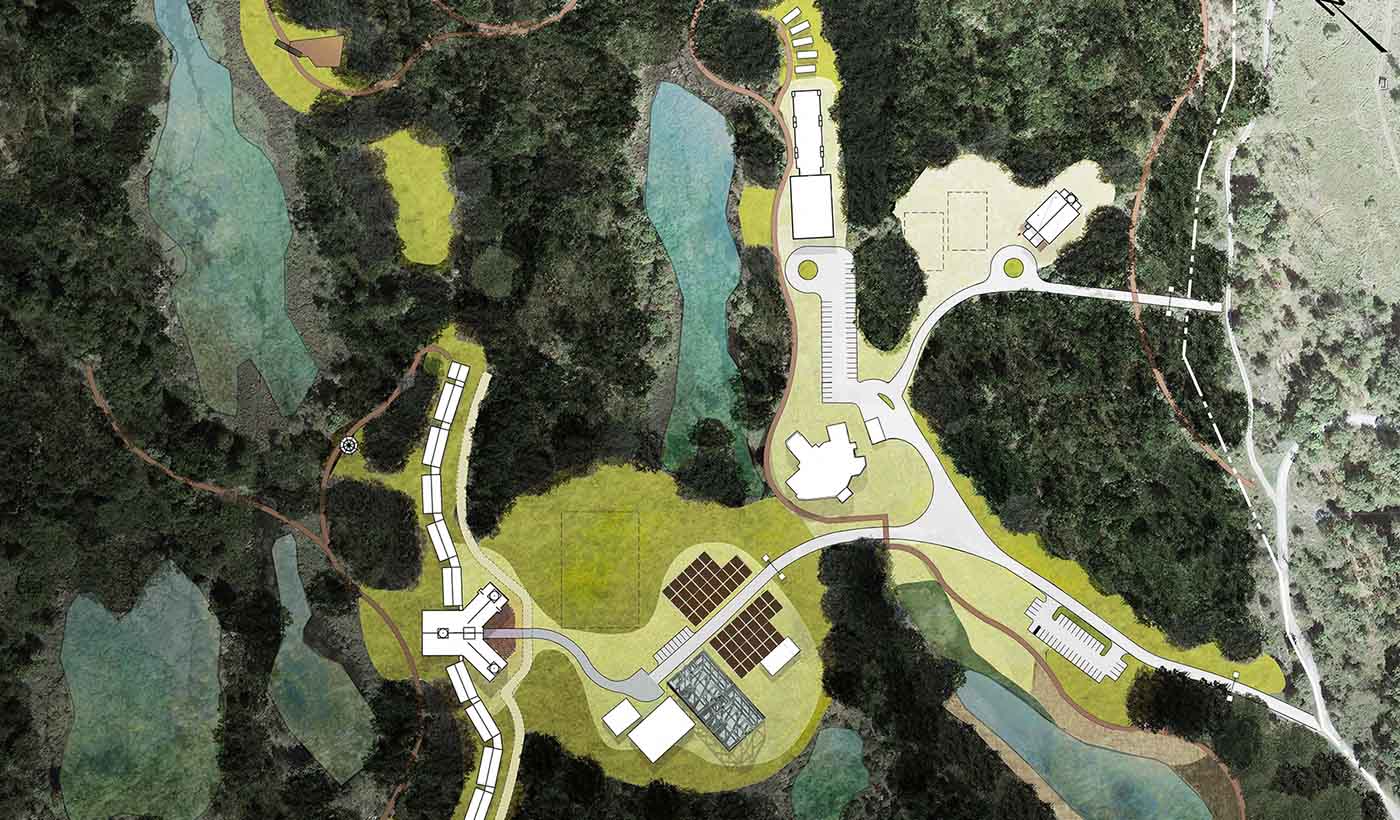
Blog Post From ancient to contemporary: How to design a monastery in the 21st century
-

Published Article Published in Innovation: Courting Disaster: The Increasing Challenges of Risk Assessment
-

Blog Post Design comeuppance: The call center gets its turn
-

Published Article Published in WET News: Focusing on what matters to customers
-

Blog Post From the Design Quarterly: Speak up. Get involved. Shape the future.
-

Webinar Recording Saving our Suburbs (Part 2) – How to Seize Emerging Opportunities
-

Blog Post Joining America Walks opened my eyes—it’s a long path to genuine walkability for everyone
-
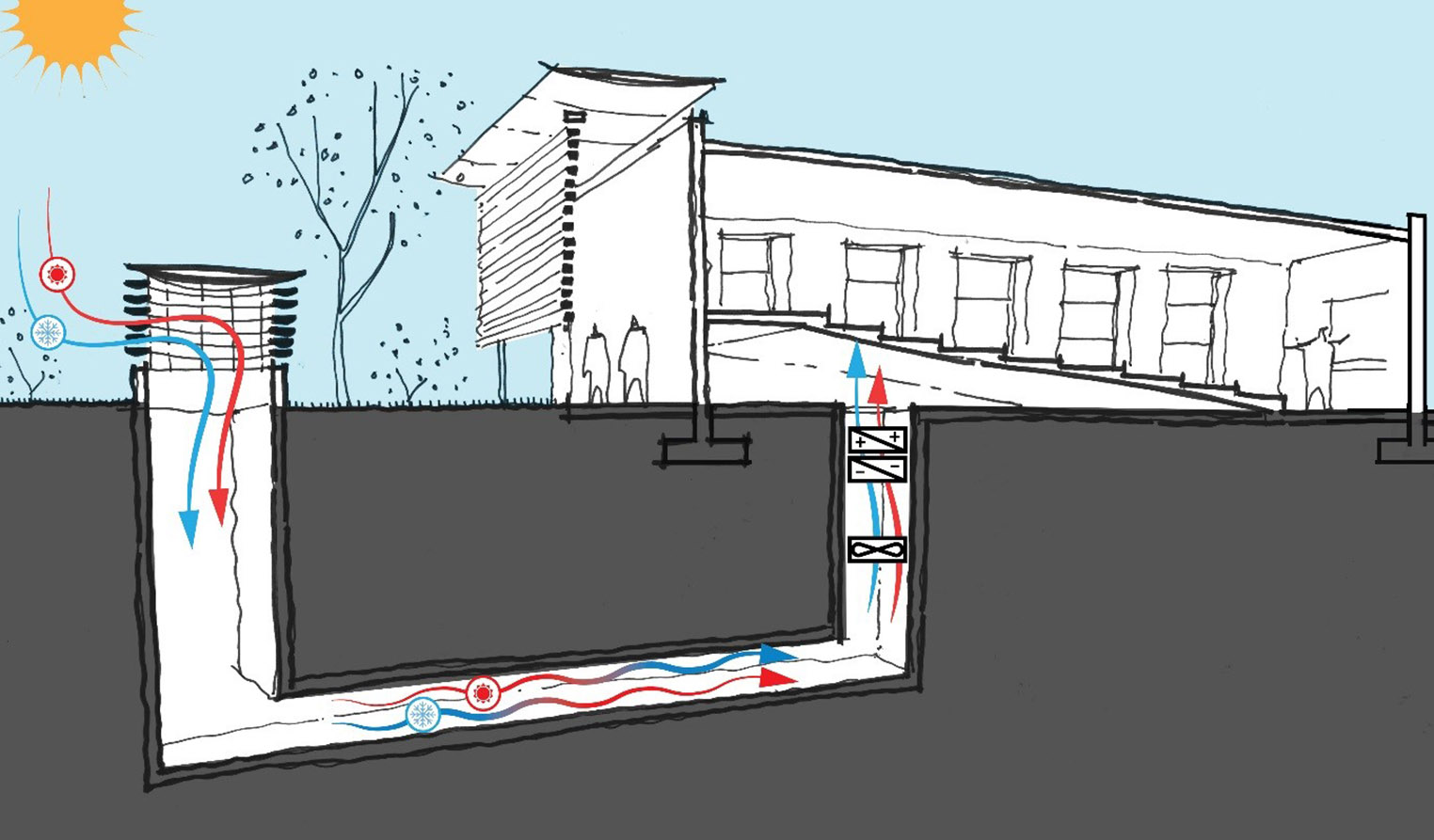
Blog Post From the Design Quarterly: Why a buildings engineer may see an earth tube in your future
-

Article Stantec offer mentoring to indigenous students
-

Blog Post How virtual reality can improve client and end-user relationships
-

Blog Post From the Design Quarterly: Creating the right collisions for higher education
-

Article Published in Parks & Rec Business: An Uphill Battle
-

Blog Post Listening at the point of care: 4 key points from the first-ever Caregivers’ Forum
-

Video Mock-up vs VR. Which is a better tool during design?
-

Video A Day in the Life of Aurel
-

Article Published: Structural engineer rides out 7.0 quake in Anchorage, then inspects for damage
-

Article The Dream Cabin: Reinventing what it’s like to sleep in a hotel room
-

Video Rethinking details with Passive House
-

Blog Post From the Design Quarterly: Landscapes that do more
-

Published Article Published in The Military Engineer: Attacking emerging contaminants at Eielson
-

Article From the Design Quarterly: A healthy future for mass timber in medical facilities
-

Published Article Published in Institute of Water: PR19 - Will it deliver a better future for water customers?
-

Blog Post Open plan 2.0: Tips for a successful transition to a new open-office design
-
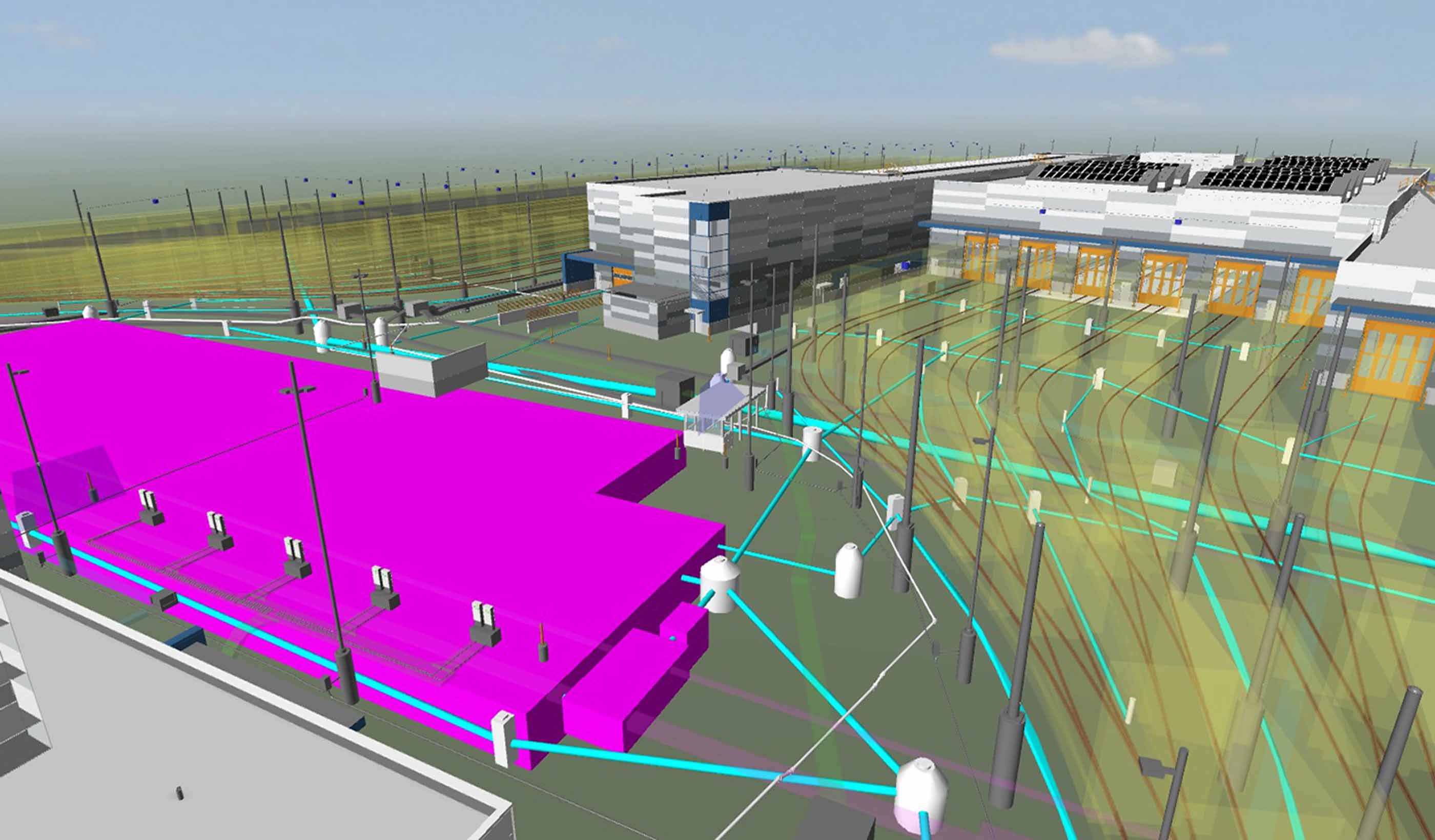
Blog Post Merging design technologies helps fast-track transit project in fast-growing Seattle area
-

Blog Post From the Design Quarterly: 7 facets of the changing airport
-

Webinar Recording Bitumen Beyond Combustion - Carbon Fibres and Uses
-

Blog Post How do we find funding for cities? It’s all about getting creative with partnerships
-

Article How modern tracking technologies can protect birds and support wind farm permitting
-

Blog Post Prefab lab systems: Concepts to optimize construction, maintenance, and flexibility
-

Blog Post Smart curtailment: the little-known battle between nature and efficient wind power
-

Blog Post Blockchaining ecosystem services to provide natural capital accounting
-

Blog Post From the Design Quarterly: Breaking down silos in healthcare education
-

Blog Post Curiosity and artistry: 6 takeaways on downtowns
-

Video Designing your future
-

Blog Post Embracing a new eDNA technology
-

Video Mental Health and Substance Use Wellness Centre at Royal Columbian Hospital
-

Blog Post The top 3 things that make managing a forest preserve successful
-

Webinar Recording Saving our Suburbs (Part 1) – How to Create Successful Suburbs
-

Blog Post From the Design Quarterly: What happens when hospitals move out?
-

Blog Post Budget 2018: A development & infrastructure view
-

Blog Post Infrastructure: The most important conversation you’re not having
-

Video Building a collaborative community in Austin
-

Blog Post Planning for life beyond HS2
-

Published Article Published: Nontraditional solutions deliver multiple benefits for municipalities
-

Blog Post Presenting Design Quarterly 04: Intersections
-

Publication Design Quarterly Issue 04 | Intersections
-

Blog Post Do you know where your recyclables are? Probably not
-

Blog Post Driving economically robust downtowns from buildings that are already there
-
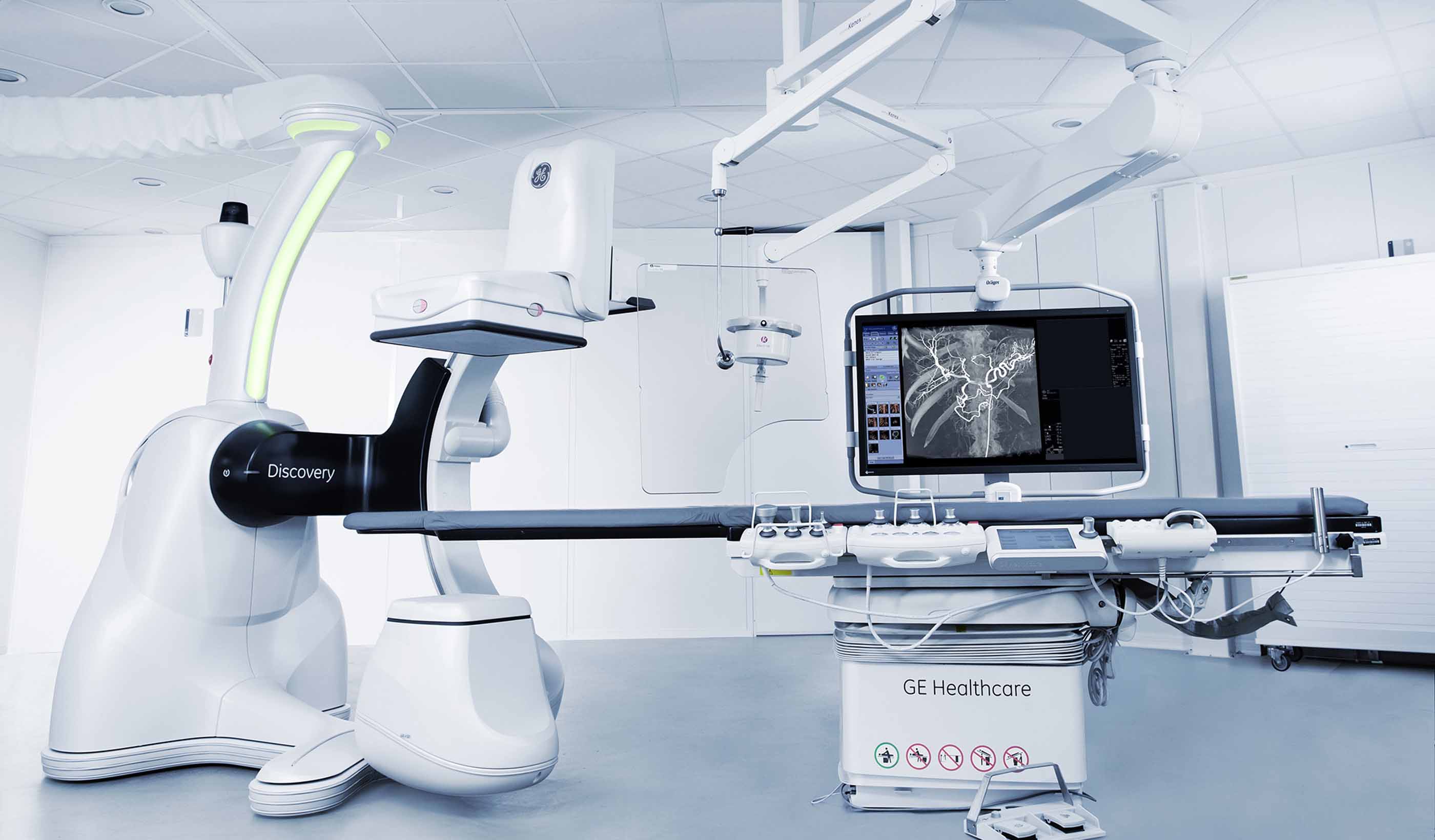
Blog Post How the right-sizing of hybrid operating rooms helps hospitals, surgeons, and patients
-

Blog Post The science of wayfinding—a formula to increase transit ridership
-

Blog Post Designing healthcare spaces for kids: Infusing a sense of exploration and curiosity
-
Blog Post Healthy light: How to design tunable or dynamic lighting to benefit patients and staff
-

Blog Post BREEAM New Construction 2018 - Part Two
-

Blog Post From the Design Quarterly: Embracing new technology to improve design
-

Blog Post 7 principles to create a get-well plan for healthcare
-

Blog Post Planning for disasters: 3 ways science can help deliver drinking water after an earthquake
-

Blog Post Are we keeping pace with climate change?
-

Published Article Published in Water Briefing Global: David Smith on the benefits of resilient megacities
-
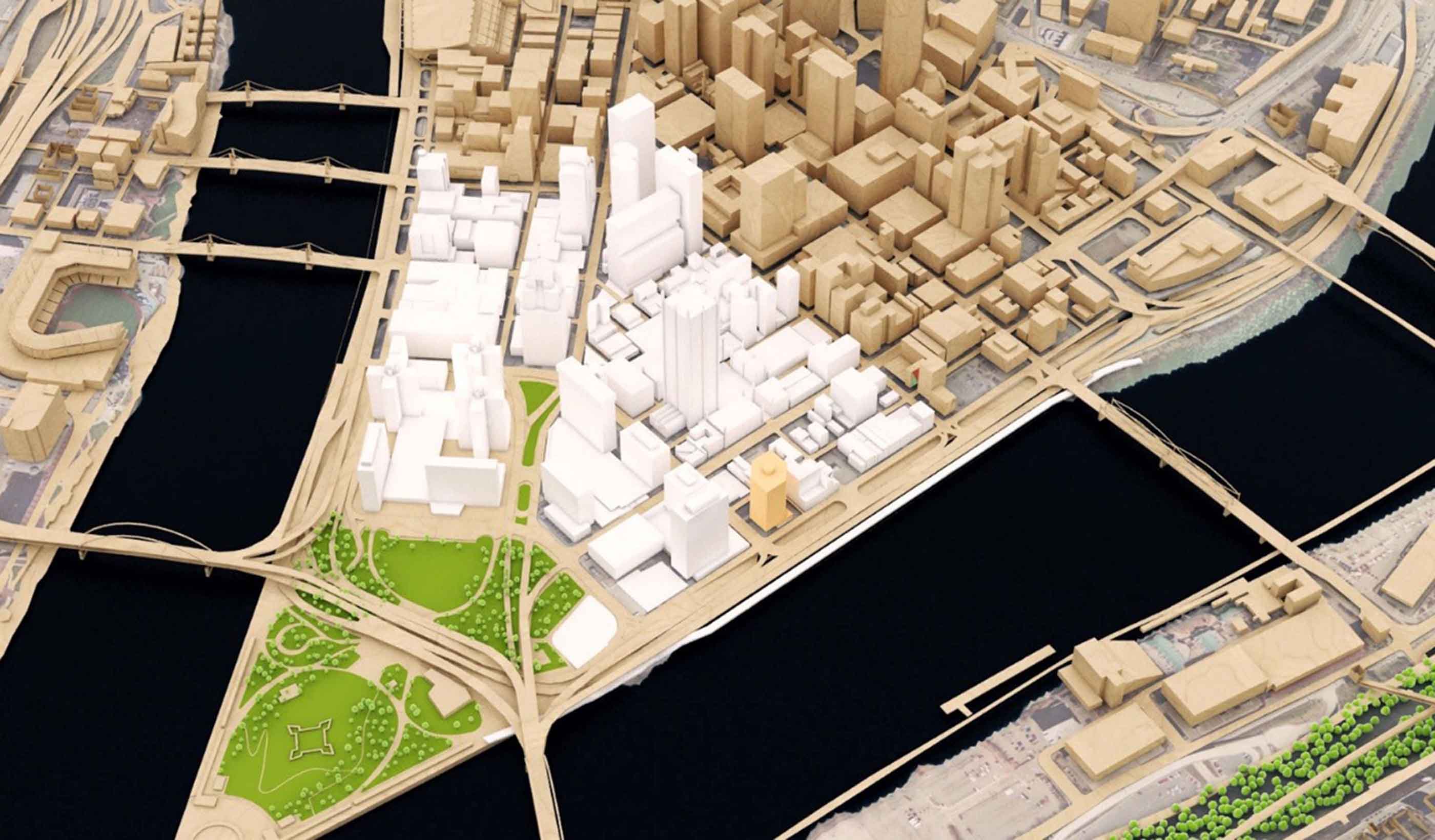
Blog Post From the Design Quarterly: Digitally illuminating a city’s utilization
-

Blog Post Roundtable: Imagining a day without water—with planning, it’s something we can avoid
-

Blog Post Fierce and fashionable: Designers turn commercial interiors materials into wearable art
-

Blog Post Corporate workplace design: 8 interiors trends to watch
-

Blog Post The charms and challenges of working on Mackinac Island include winter and water
-

Published Article Published in Seattle DJC: OSU football center opts for flexible lighting scheme
-

Blog Post Artificial Intelligence: One answer to reducing greenhouse gases at industrial facilities
-

Blog Post BREEAM New Construction 2018 - Part One
-

Published Article Published in LD+A Magazine: Circadian in the Workplace: Does it make sense
-

Blog Post From the Design Quarterly: Bringing an urban campus together for the better
-

Published Article Published in Ontario Technologist Magazine: Wood Pole Design for Overhead
-

Blog Post World Architecture Day: How do people and places impact global design decisions?
-

Blog Post Holistic stormwater management often requires more than ponds and rain gardens
-

Blog Post 4 things to consider when tendering a stream project
-

Blog Post Stantec: Our evolution from 1 person to a global firm
-
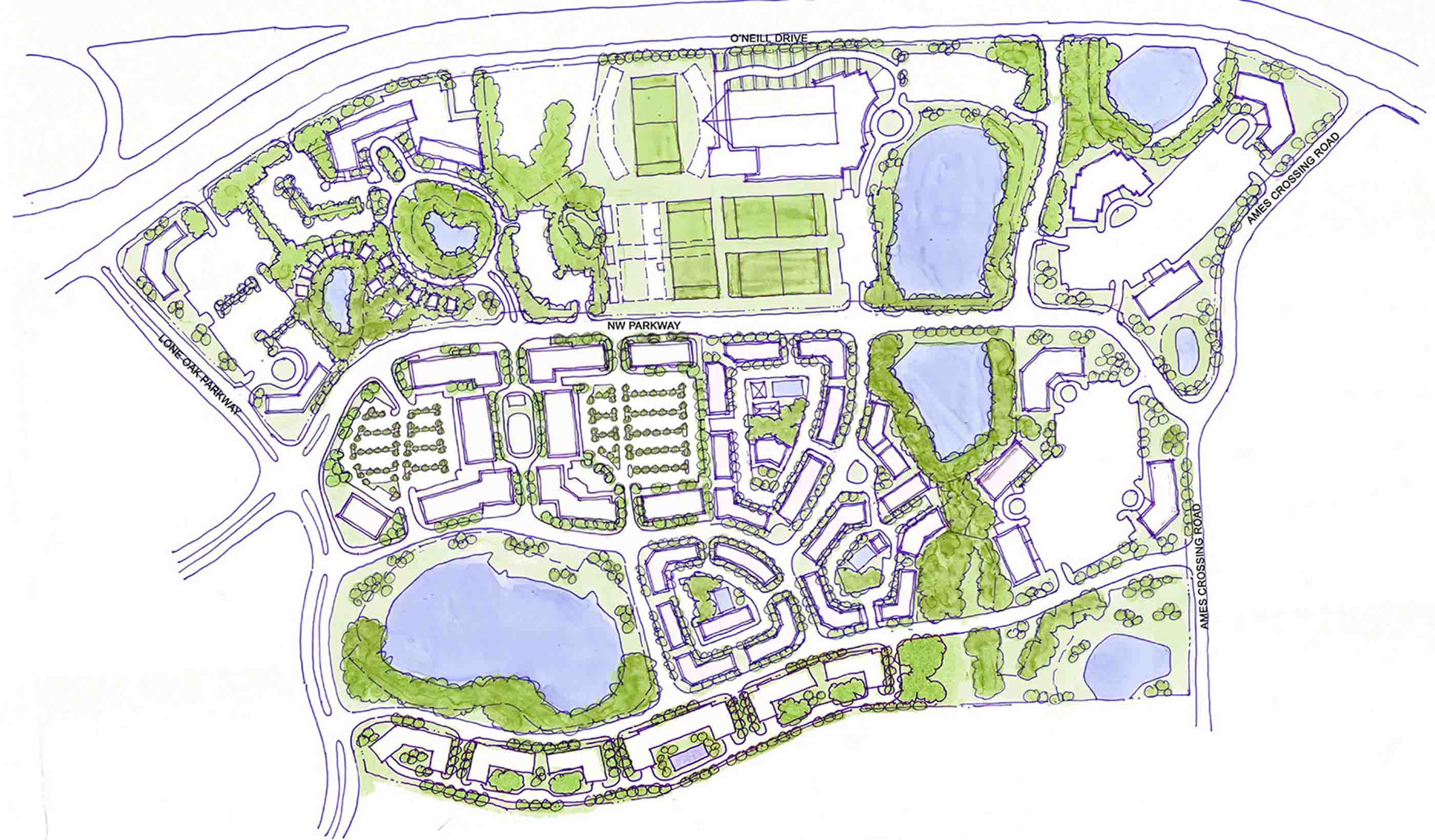
Blog Post Using an environmental review tool to yield planning and economic development benefits
-

Blog Post 5 reasons to rent a bike instead of a car during your next business trip
-

Blog Post Counting every space: What parking inventories tell us about cities
-

Blog Post Wildfire recovery: Creating a resilient home for outdoor education from ashes
-

Blog Post National Preparedness Month: Two things you can do now to protect you and your family
-

Video Stantec Summer Internship Program 2018
-

Blog Post Why the Avenir Centre stands as Raven Spanier’s legacy
-

Blog Post From the Design Quarterly: Old idea, new form—makerspaces make it big
-
![[With Video] Project Impossible? Not when you have the right team!](/content/dam/stantec/images/ideas/blogs/007/with-video-project-impossible-1.jpg)
Blog Post [With Video] Project Impossible? Not when you have the right team!
-

Blog Post Nature is a patient, not a machine: Looking at stream restoration through the medical lens
-

Video Libraries & Learning Commons, Part 4
-

Blog Post A quest to find innovative technologies for managing deteriorating water infrastructure
-

Blog Post How to plan for affordable, supportable utility rates
-

Published Article Published: TAP IN - ATCO's Heartland Industrial Water System
-

Video Advancing our communities through increased mobility
-

Blog Post From the Design Quarterly: Forecasting change for baby boomers and millennials
-

Video Sustainable solutions to flooding in NZ
-

Blog Post Planning for the ups and downs of the general aviation market
-

Video Two Words: One Choice
-

Blog Post Clean Air Zones - driving the wrong way?
-

Blog Post Coastal crisis: We’re running out of sand along Florida’s beaches—what’s the solution?
-

Blog Post EcoDriving can save you time, money, and effort—all while reducing your carbon footprint
-

Blog Post Designing in “light” of circadian support: 6 strategies for commercial spaces
-

Blog Post Presenting Design Quarterly 03: Technology driving change
-

Publication Design Quarterly Issue 03 | Technology Driving Change
-

Blog Post Engineering the impossible at Nunavut’s Iqaluit Airport
-

Published Article Small dam, big task
-

Blog Post 7 ways to tell if a stream restoration project is successful
-

Blog Post Putting Places First
-

Published Article Published: Making The Most Of What You Have - Optimisation Of Assets In Water And Energy
-

Blog Post What role should affordability play when utilities set their rates?
-
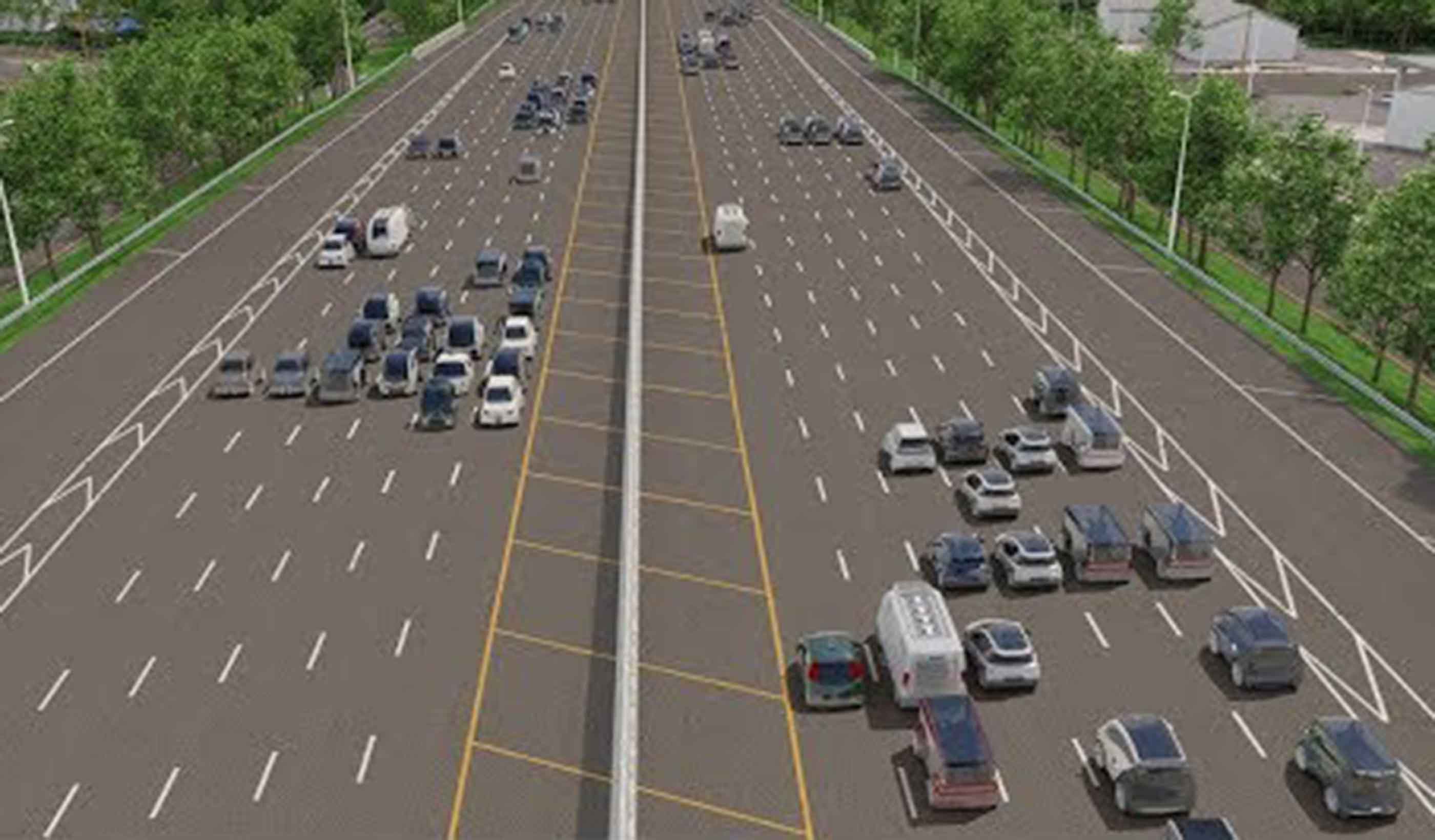
Video The Future of Freeways: The Impact of Connected and Automated Vehicles
-

Blog Post The Road to Zero or the Road to Roadworks?
-

Blog Post 5 steps for successful delivery of transmission and distribution projects
-

Article Need to keep your community's bridges safe? Stantec is creating an app for that.
-

Blog Post Creating “in-between” spaces in research facilities
-

Blog Post From the Design Quarterly: Culture 2.0 in the workplace
-

Video David Dixon discusses the next generation of urban places at NZPI 2018
-

Blog Post The Water Bar: Serving up conversations about water supply and use
-
![[With Video] Living WELL: Designing a new home for hundreds with health at the heart](/content/dam/stantec/images/ideas/blogs/004/with-video-living-well-1.jpg)
Blog Post [With Video] Living WELL: Designing a new home for hundreds with health at the heart
-

Published Article Mine design from the “inside out”
-

Blog Post Five traits a surveyor must have to help a client find success
-

Article Stantec at HydroVision: Three leaders reflect on past conferences
-

Published Article Published in IEEE: Mike Voll on the David Johnston R+T Park Microgrid
-

Blog Post The perfect battery: Pumped storage hydropower is reliable, rechargeable, and clean
-

Blog Post Top 5 things to keep in mind as you seek funding for your capital projects
-

Published Article Published in Florida Water Resources Journal: Keys to Planning, Designing, and Permitting Resilient Coastal Restoration Projects
-

Article NPR's Planet Money features the world's biggest battery
-
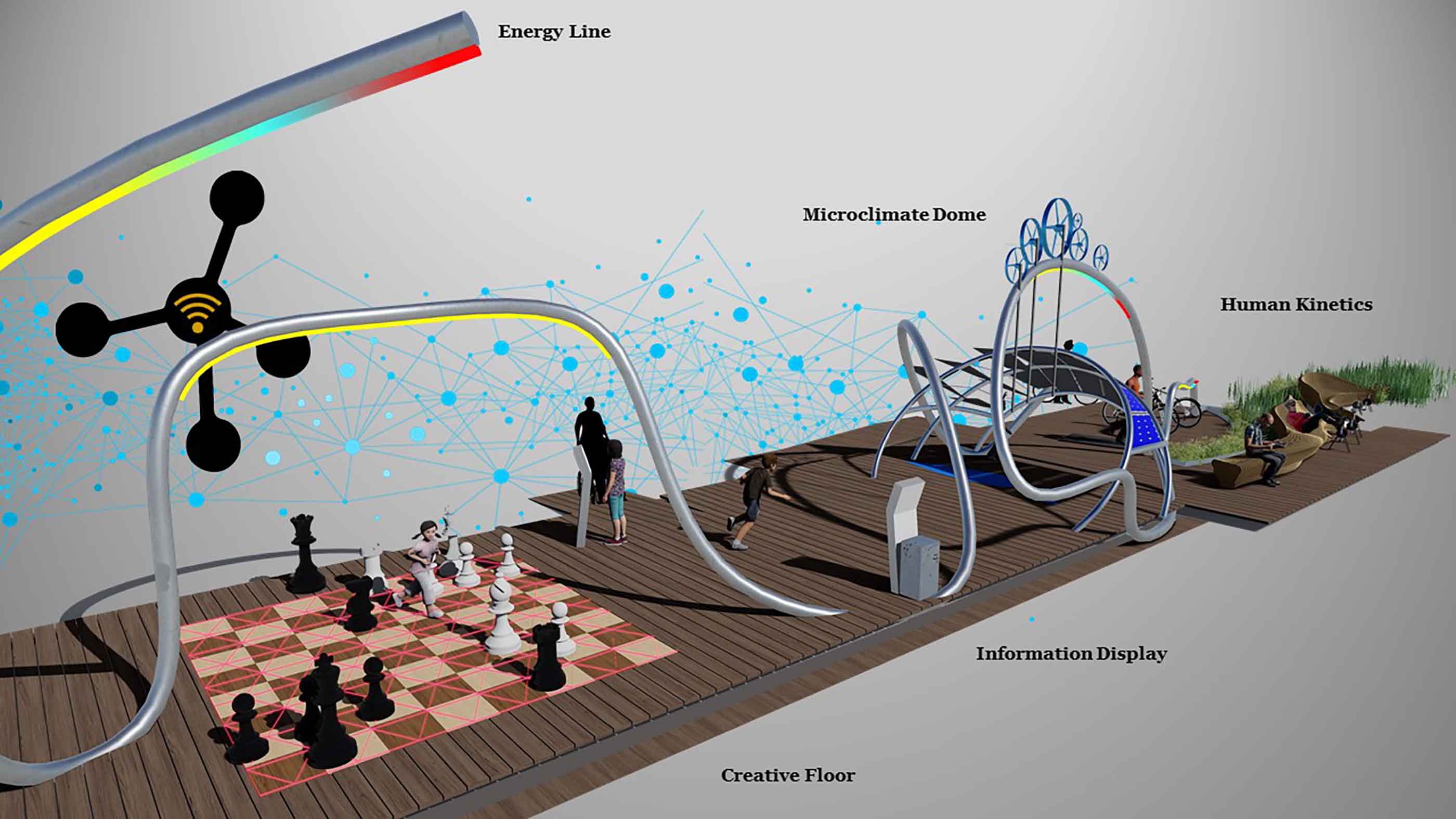
Article Building urban energy resilience through education: The E-Pop Park
-

Article International Downtown Association Report: The Value of U.S. Downtowns and Center Cities
-

Blog Post From the Design Quarterly: Bridging cultures to create buildings for unique populations
-

Blog Post The flying menace coming to a city near you!
-

Blog Post In the battle of suburbs vs. cities, could both be winning?
-

Video Libraries & Learning Commons, Part 3
-
![[With Video] Your community needs a BMX track—here’s why](/content/dam/stantec/images/ideas/blogs/013/with-video-community-needs-a-bmx-track-1.jpg)
Blog Post [With Video] Your community needs a BMX track—here’s why
-

Published Article Published: New Grit Recovery System Installed at Edmonton's Gold Bar WWTP
-

Blog Post Microgrids: An energy solution for small towns with big aspirations
-

Blog Post It’s important to focus on the positives of major projects. How do you do it?
-

Blog Post Six ways to improve your greenhouse gas verification process (Part 3)
-

Blog Post Why your office needs a makerspace—it’s about investing in the creative workplace
-

From the Design Quarterly: Healthy neighbors
-

Blog Post Creating a new paradigm for traffic safety—trade the car for other transit options
-

Blog Post New housing numbers and new population projections: all crystal clear now?
-
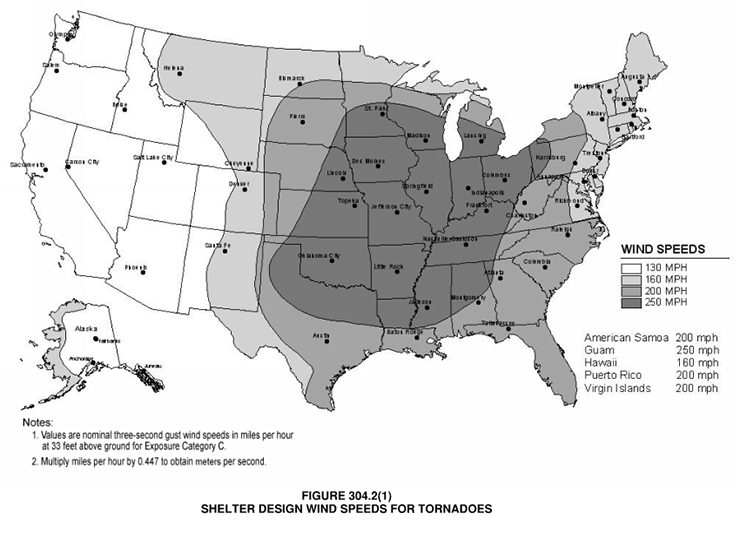
Blog Post 3 things to consider when designing a storm shelter for your school
-

Blog Post Living shorelines: Recovery, restoration, and resiliency after Hurricane Sandy
-

Published Article Clear and clean
-

Blog Post Four questions we must answer to design truly smart cities
-

Blog Post Mental health: How architects can help direct a path toward recovery
-

Blog Post How do you pick the right greenhouse gas verification body for your project? (Part 1)
-

Blog Post From the Design Quarterly: Switching on the off-season
-

Blog Post Looking back at Infrastructure Week: We have our marching orders—educate and advocate
-

Blog Post How can we improve combined sewer overflow reporting? We need a smarter approach
-
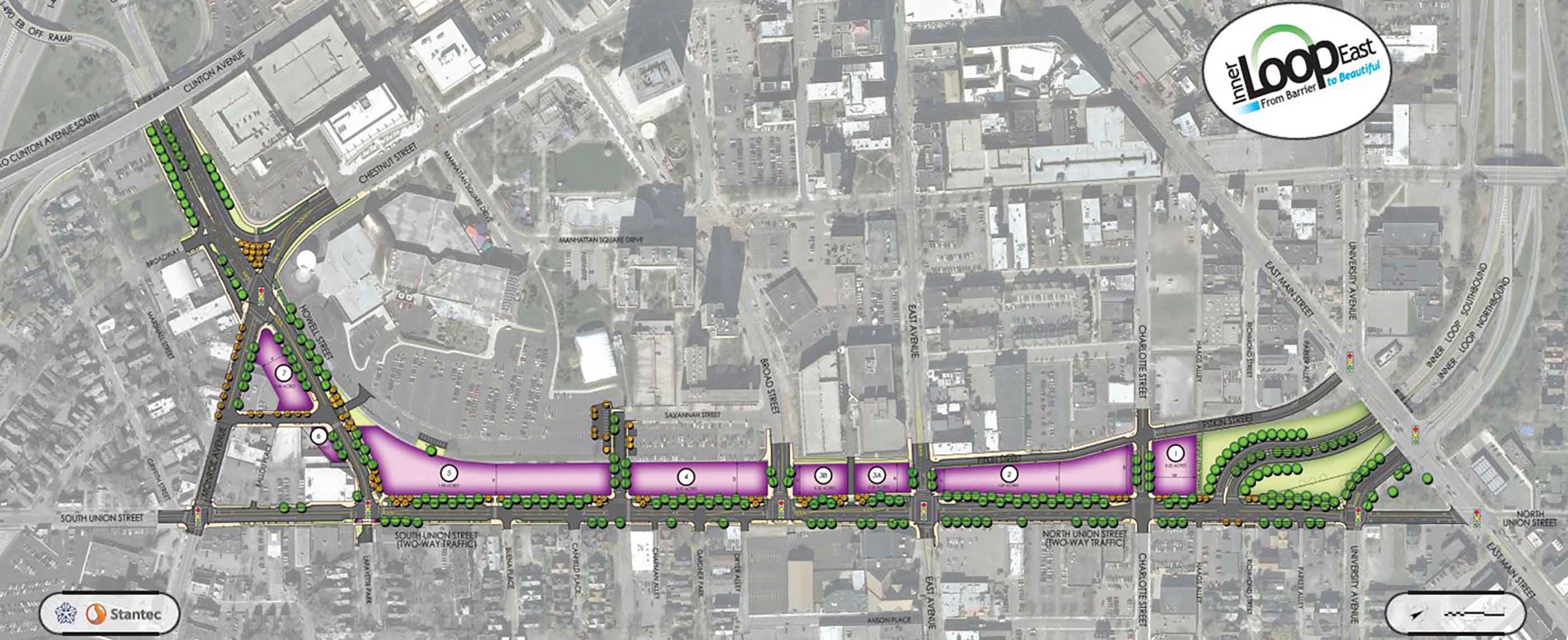
Blog Post Road kill: the creative destruction of an urban expressway reclaims a city’s soul
-

Blog Post What does affordable housing look like in a thriving metro area?
-

Blog Post From the Design Quarterly: Incubating campus cool
-

Video Preserving affordable housing in Denver
-
Blog Post Seeing the future: Harnessing the power of virtual reality for community engagement
-

Blog Post 5 ways to make healthcare waiting rooms more functional and comfortable
-

Published Article Planning on the Cellular Level - Planning Magazine, May 2018
-

Publication Design Quarterly Issue 02 | Culture by Design
-

Blog Post Molecular branding: Designing to support innovation in cancer therapies
-

Blog Post What happens when 4,000 people commit to Getting to Zero?
-

Blog Post Earth day 2018: actions not words
-

Video Libraries & Learning Commons, Part 2
-

Blog Post Sustainability goes beyond zero carbon—it’s about creating greater wellness for tenants
-

Blog Post In the design realm, you must have the client’s trust before you can innovate
-

Blog Post Earth Day 2018: end plastic pollution
-

Blog Post Urban Places Idea Lab: Tweaking plans, designs, and sustainability strategies
-

Blog Post Hard Hats with Heart: Giving with the community’s health in mind
-

Published Article Collaboration Re-Defined: Positioning for Success at PR19
-

Video Mapping the depths in Holland
-

Published Article Sustainability drives dam development
-

Blog Post Planning for 100: Looking beyond the horizon of zero-net-energy buildings
-

Blog Post European Union embraces low-carbon, circular economy: How will it get there?
-

Blog Post What makes a boutique hotel stand out from the rest?
-

Published Article Conveying water in steep heights: Solutions for a hydropower project in the Andes Mountains
-

Blog Post Connecting Canada from coast to coast to coast—completing a 60-year vision
-

Video Adding value to our projects with 3D printing
-

Blog Post Embracing the challenge in a new era of project management and delivery
-

Article Rebecca Keehner, advancing interior design through art and science
-

Blog Post Celebrating World Water Day: What will it take to secure our water future?
-

Blog Post Economics of Water Series: Changing landscapes in the global water industry
-

Blog Post Power poverty: the new paradigm for social and economic inequality of electric vehicles
-
Blog Post When is it time to rip off the band-aid and really plan for healthcare facility power?
-

Blog Post What are cultural heritage landscapes? Why are they important?
-

Blog Post Evolve or die: Why change is necessary in the transmission and distribution world
-

Video Libraries & Learning Commons, Part 1
-

Navigating the draft revised National Planning Policy Framework
-

Blog Post Water rich or water poor? A quantitative tool can help water resources planners
-

Video Designing for STEM Education
-

From the Design Quarterly: Living small means the city is your living room
-

Blog Post How can utilities help solve the pollinator crisis?
-

Blog Post A new way to approach community involvement for brownfield projects
-
Blog Post Should UV light be a weapon in our infection-control arsenal inside hospitals?
-

Blog Post What does Value of Downtowns report tell us about tomorrow’s cities and regions? (Part 2)
-

Blog Post A powerful lesson: Selecting a consultant based on price alone is a risky decision
-

Blog Post Urban Greening and the Draft New London Plan
-

Video It takes a village: Re-envisioning a main street in Rolesville, NC
-
Published Article Read a free chapter of Suburban Remix, edited by Jason Beske & David Dixon
-

Blog Post Boxed in by changing markets, 20th-century suburbs learn how to reinvent themselves
-

Blog Post World Wetlands Day 2018
-

Blog Post What does The Value of Downtowns report tell us about today’s cities? (Part 1)
-

Blog Post Traffic management and preparing a city for the big game
-

Blog Post From the Design Quarterly: Where’s my trashcan?
-

Blog Post Give your HQ some heart: Creating branded workplaces
-

Blog Post North American electric utilities: Where they’ve been and where they’re going
-

Blog Post King Cove Road: Balancing the needs of people and nature in remote Alaska
-

Blog Post The healthy garden city
-

Publication Issue 01 | The Sustainable City
-

Blog Post A bridge to the future: Direct-fixation rail helps Denver commuter project span challenges
-

Blog Post We can help prevent hospital-acquired infections during renovation
-

Publication Design Quarterly Issue 01 | The Sustainable City
-

Blog Post 8 ways to raise expectations for commercial drone operators
-

Blog Post Autonomous vehicles could ruin our downtowns. But not if we act now
-

Blog Post Digging Deep into Data — How cell phone activity data enhances transportation planning
-

Blog Post Observations from the middle seat: Why we’re feeling the squeeze when we fly
-

Blog Post PFAS 101: What you need to know about perfluoroalkylated substances—and why
-

Video The 100m-tall archaeologist
-

Blog Post The renovation of iconic Wrigley Field is an example of large-scale building recycling
-

Blog Post Economics of Water Series: Extreme weather—resiliency, planning, and preparedness
-

Published Article UX Rules! How Interactive 3-D Reality Systems Enhance Client Engagement
-

Publication Stantec's Hard Rock Miner's Handbook
-

Blog Post Retail is not dead: The omnichannel revolution is just changing how it works
-
Blog Post Economics of Water Series: Extreme weather—after the hurricanes
-

Blog Post Climate change from the front lines: How the conversation is evolving in the Arctic
-

Article Early hydrogeological studies critical to mining success
-

Blog Post The challenges of post-decision monitoring in EIAs
-

Blog Post Garden cities: lighter, faster, cheaper
-

Blog Post Can Caribbean islands modernize their way to affordable, resilient energy grids?
-

Blog Post Who says non-renewable resource projects can’t deliver sustainable economic development?
-

Blog Post Infrastructure investments pay off long term; hydropower is great place to start
-

Blog Post Indigenous healthcare facility design, a conversation (Part 2)
-

Blog Post Indigenous healthcare facility design, a conversation (Part 1)
-

Blog Post Play on: How to get the most out of your sports field with artificial turf
-

Blog Post Resurrecting a community centre
-

Blog Post Garden cities & movement: achieving 'good growth'
-

Blog Post Healthcare design that cares for the whole child
-

Video A world outside the classroom
-

Published Article The future of American hydropower
-

Blog Post Can a city’s downtown be its portal to prosperity?
-

Blog Post Don’t think big box stores can be trailblazers for sustainability? Think again.
-

Blog Post Keeping an eagle eye on sustainable bridge design
-

Blog Post Housing need: what can we learn from the new CLG proposals?
-

Published Article Seychelles invests in future water security
-

Video The first/last mile problem
-

Blog Post 5 elements for a healthy medical lounge
-
Blog Post Why your community needs its own Complete Streets design guidelines
-
Blog Post Will membrane aerated bioreactors (MABR) change how we treat wastewater?
-

Blog Post Turning land-use restrictions into something positive
-

Blog Post How technology will disrupt long-term senior care
-
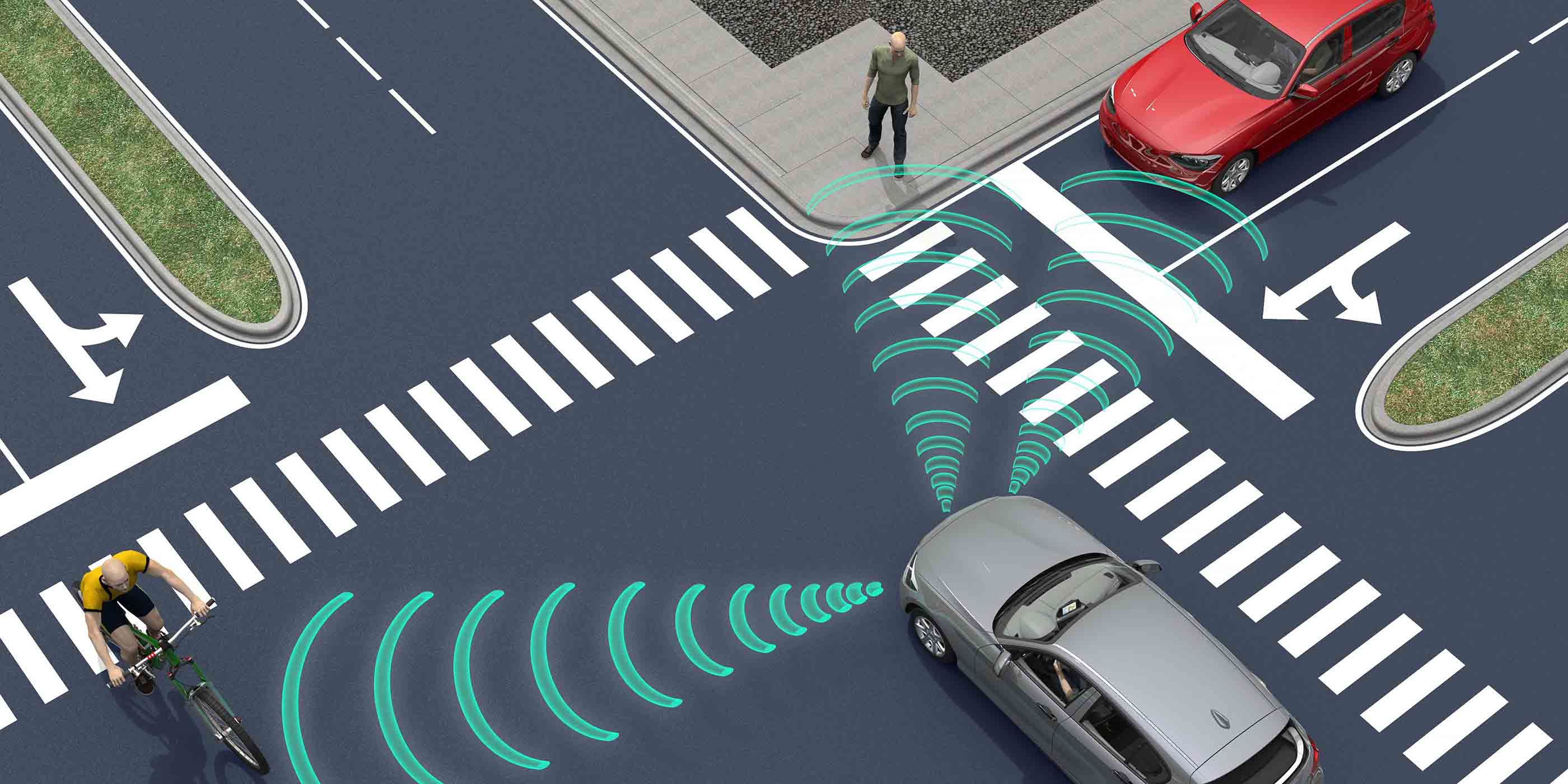
Blog Post Vehicle for change
-

Video The future of higher ed
-

Blog Post Planning policy challenges of the garden city model
-

The changing face of freight and logistics
-

Blog Post What makes a successful study space in an academic building?
-

Blog Post Dredging waterways: Turning muddy muck into something sustainable
-

Article Disrupting species identification: eDNA and a new way to identify organisms in the field
-

Blog Post Creating communities from defunct suburban malls
-
Blog Post Can you do your client's job?
-

Blog Post Digging deep into our geotechnical history across Canada
-

Blog Post When net positive is business plus
-

Blog Post Hooked on classics: Why do we still study classical architecture?
-

Blog Post 4 strategies to get the most from your project manager or owner’s representative
-

Blog Post What are your project delivery options for horizontal construction?
-

Video Engineered with old tires
-

Blog Post Turning toasty exhaust air into usable heat—That’s sustainability!
-

Blog Post The future of school design: Net Zero should be the norm
-

Blog Post Mind the gap: A translational approach to research and medicine
-

Blog Post Opening day at Wrigley Field: A behind-the-scenes view
-

Blog Post The digital utility is within our reach
-

Blog Post How the Taussig Cancer Center is making the patient experience number one
-

Blog Post The quest for knowledge: How Stantec research empowers our people to better serve clients
-

Blog Post Communicating cars: Understanding the road to autonomous vehicles
-

Published Article The next big disruption is here
-

Video Engineers on rope
-

Blog Post Denver’s Metro is on the road to a utility of the future
-

Blog Post Decoding Canada’s Climate Innovation Program
-

Blog Post City building: How to use place-making to get intensification right
-

Article Determining infrastructure life cycle costs with the CLIC tool
-

Video Don't look down
-

Blog Post A US Infrastructure Report Card 2017 preview
-

Article Turning the tide: Helping shoreline communities create ecologically balanced seawalls
-

Blog Post Turning transport planning on its head: part II
-

Blog Post Planting for the future: Enhancing communities with a thriving tree canopy
-

Blog Post Embracing the chill: Turning winter into a design asset
-

Whitepaper The housing white paper: a review
-

Blog Post Ensuring safety when there is no standard
-

Article Inside a micro-unit: Living small, sharing more, and creating affordable urban housing
-

Blog Post Public realm for 21st-century cities
-

Blog Post Creating a big STEAM impact on a small budget
-

Blog Post Trends affecting the corporate workplace
-

Blog Post Five must-have rooms in the workplace of the future
-

Video How bridges stay healthy
-

Blog Post Turning transport planning on its head: part I
-

Blog Post How we found hidden energy savings in water treatment plants
-

Blog Post The Jane Jacobs conundrum (Part 1): 3 urban trends driving economic inequality
-

Blog Post Brexit: an environmental opportunity?
-

Blog Post Why Stantec is invested in pipeline safety, efficiency, and integrity
-

Published Article Saving Chicago from future flooding
-

Blog Post What's the impact of school design on student learning?
-

Blog Post How to create faster, cheaper 4D construction animation
-
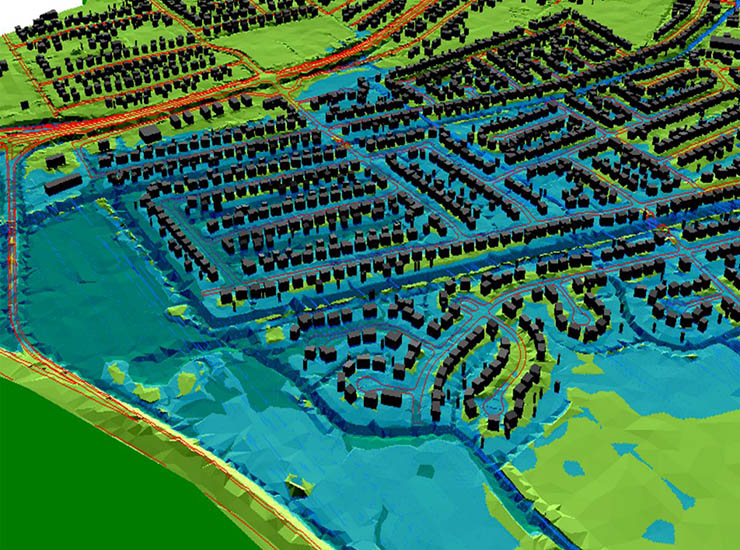
Blog Post Is your levee at risk? 3 ways to keep your community safe
-
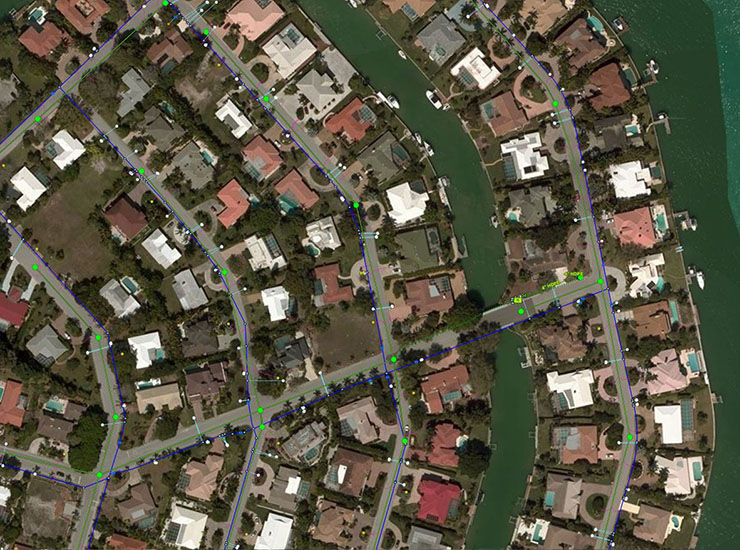
Blog Post Putting geographic information systems data online to make city life easier
-

4 key steps toward a resilient airport
-

Blog Post If you build it, they will come: Designing a self-sustaining trout stream
-

Blog Post Landscape architects: On the front lines of the battle for resilience
-

Blog Post The Airbus A380: How it's influencing airport design
-

Blog Post Transforming design through virtual reality
-

Published Article Beyond water management
-
Published Article The importance of water according to mining experts
-
Blog Post Finding a solution: Will sea level rise still be a concern in 50 years?
-

Blog Post How long is an airport’s runway?
-

Published Article Stormy waters—managing urban flooding in the Middle East
-

Blog Post Explaining the case for infrastructure projects
-

Blog Post Making a case for living small and sharing more in urban areas
-

Blog Post Airport runway lights: What are they all for?
-

Video A dream come true in Harbour Landing
-

Blog Post Airport runways: What do those big numbers mean?
-

Blog Post How can we design adaptable buildings?
-

Blog Post Transit-Oriented Development (TOD): It’s not just about the station
-

Blog Post Ditching the rink to skate on a rolling ribbon of ice
-

Blog Post Top 5 tips for fast-track site development
-

Blog Post How weeks spent in the field led to the largest freshwater turtle recovery in the US
-

Blog Post The battle of development: Urban core or suburbs?
-
Blog Post Why users enjoying the public spaces we design is the best reward
-

Interactive How can a tiny park pack a big punch?
-

Interactive How to manage flooding while improving the cityscape
-
Interactive Which remote sensing technology is right for you?
-

Interactive Designing interiors with non-toxic materials
-

Interactive 9 breakthrough technologies for tackling climate change
-

Interactive You have an energy transition or climate action plan, but is it integrated?
-

Interactive Forever chemicals: Understanding the risks of PFAS
-

Interactive eDNA: Delivering power sector projects while keeping rare species safe
-

Interactive Can ecosytem restoration help save the planet?
-
Interactive What does ecosystem restoration look like?
-

Interactive Get to know the digital twin
-

Interactive Is mass timber a viable option for your next building project?
-

Interactive City-scale amenities give Sacramento its waterfront back
-

Interactive 6 keys to designing sustainable infrastructure in developing countries
-

Stantec ERA Issue 7 | The Big Ideas Issue
-

Publication Stantec ERA Issue 8 | The Extreme Weather Issue
-

Publication Stantec ERA Issue 9 | The Water Issue
-

Stantec ERA Issue 6 | The Climate Change Issue
-

Publication Stantec ERA Issue 04 | The Energy Transition Issue
-

Stantec ERA Issue 5 | The Sustainability Issue
-

Interactive Microgrids can provide resiliency during extreme weather events
-
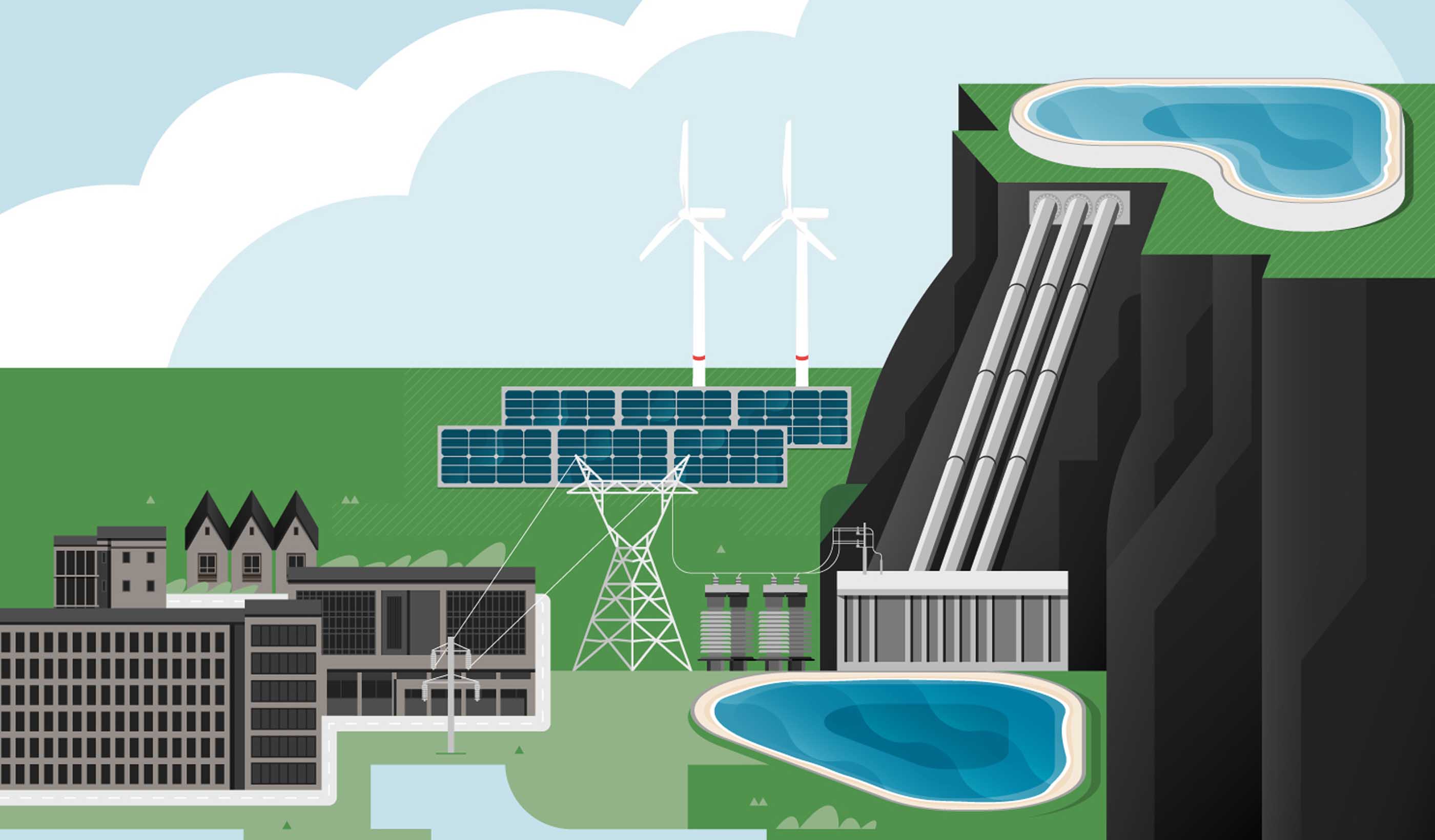
Interactive Can pumped storage fuel a more sustainable future?
-

How can biogas power our communities more sustainably?
-

Interactive How can corporate values help to create more sustainable communities?
-

Interactive Wastewater Thermal Hydrolysis Processing THP
-

Interactive 4 guiding principles to secure our water future
-

Interactive What’s your stormwater issue?
-

Interactive 6 approaches to water management that can help address climate change
-

Interactive Preserving the School in the Trees
-

Interactive A new stage for hands-on education
-

Interactive Think local: meet microgrids
-

Interactive Powering our communities with offshore wind energy
-
Interactive Panama Canal Digital Twin
-

Interactive Why is everyone talking about hydrogen?
-

Interactive Hardening the grid
-

Interactive City Transit
-
Interactive Explore The Autonomous Vehicle City
-
Interactive Quiz: Is your community automated vehicle ready?
-

Interactive What is microtransit? How to integrate more riders into a transit system
-

Interactive A net zero world needs mining
-

Interactive How VR and AR are transforming the world of energy and resources
-

Interactive When was the last time your mine had a check-up?
-

Interactive 6 Ways to Meet Your ESG Goals Through Nature
-

Interactive Planning healthcare facilities with Playmobil figures and 3D printers
-

Interactive Coastal resilience by the numbers
-

Interactive Protecting the Gulf: A Story of Coastal Resilience
-

Interactive Frontiers in workplace wellness
-

Interactive Getting back to the office: Our COVID-19 research highlights
-

Interactive The smart, flexible workplace
-

Interactive How smart wind farm curtailment can save bats and optimize energy gains
-

Interactive United for Infrastructure: Exploring our changing urban landscape
-

Interactive How will you survive the next big disaster?
-

Interactive The Stantec Idea Hackathon: Big ideas for our biggest challenges
-
Interactive Smart Cities Quiz
-

Interactive The 7 best practices of highly effective smart cities
-

Interactive Ecosystem Restoration: Solutions for global problems
-

Interactive The United Nations Sustainable Development Goals (SDGs) can help you
-

Interactive Making urban places work for people
-

Interactive What does it take to turn around a declining mall?
-

Interactive Finding freedom and balance in the new office
-

Interactive What does the hotel comeback look like?
-

Interactive 18 environmental DNA applications to help achieve nature positive goals
-

Interactive Changing field reporting through technology
-

Publication Stantec ERA Issue 10 | The Carbon Issue
-

Interactive How can we successfully integrate renewables into the energy grid?
-

Interactive What's in your EV battery?
-

Interactive Rethinking the life of a mine through mine closure planning
-

Interactive Mobility hubs are on the way
-

Interactive Stantec's Innovation Office: Nurturing ideas to support our communities
-

Interactive Building Decarbonization Design Guide
-

Interactive What does decarbonizing mining look like?
-

Interactive What we talk about when we talk about the energy transition



![[With Video] Transitioning to recovery while preparing for a second wave of COVID-19 cases](/content/dam/stantec/images/ideas/blogs/014/with-video-transitioning-to-recovery-1.JPG)







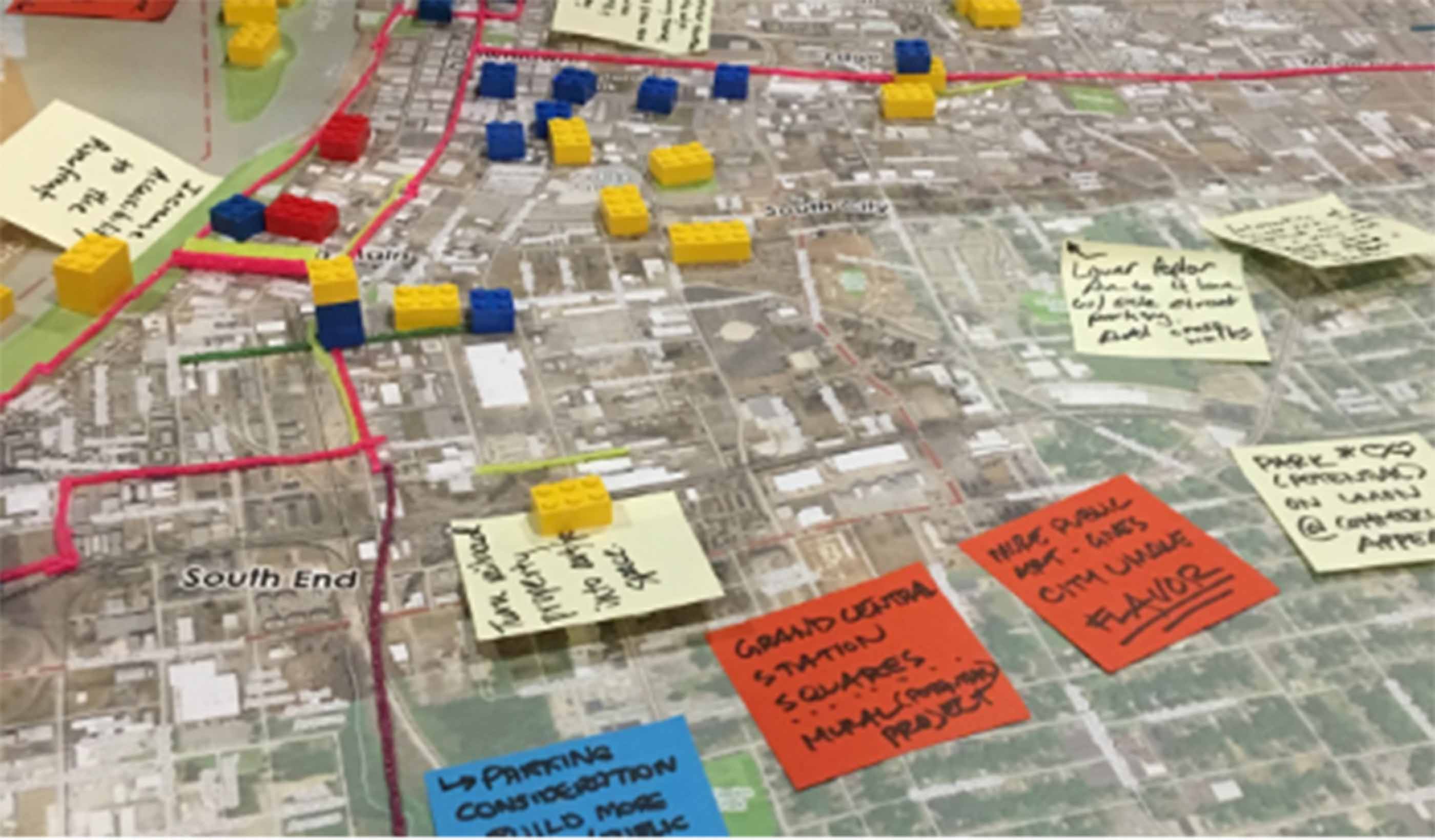
![[With Video] 5 ways to design a transit maintenance facility that’s also a people place](/content/dam/stantec/images/ideas/blogs/004/whitby-rail-maintenance-1.JPG)











